Chinese Journal of Organic Chemistry ›› 2021, Vol. 41 ›› Issue (7): 2800-2809.DOI: 10.6023/cjoc202102045 Previous Articles Next Articles
ARTICLES
王金艳a, 董黎颖a, 刘雅妮a, 陈西同a, 马艳楠a, 尹昊a, 杜姗姗b,*( ), 齐昀坤a,*(
), 齐昀坤a,*( ), 王克威a
), 王克威a
收稿日期:2021-02-24
修回日期:2021-03-24
发布日期:2021-04-12
通讯作者:
杜姗姗, 齐昀坤
作者简介:基金资助:
Jinyan Wanga, Liying Donga, Ya'ni Liua, Xitong Chena, Yannan Maa, Hao Yina, Shanshan Dub( ), Yunkun Qia(
), Yunkun Qia( ), Kewei Wanga
), Kewei Wanga
Received:2021-02-24
Revised:2021-03-24
Published:2021-04-12
Contact:
Shanshan Du, Yunkun Qi
Supported by:Share
Jinyan Wang, Liying Dong, Ya'ni Liu, Xitong Chen, Yannan Ma, Hao Yin, Shanshan Du, Yunkun Qi, Kewei Wang. Efficient Synthesis and Oxidative Folding Studies of Centipede Toxin RhTx[J]. Chinese Journal of Organic Chemistry, 2021, 41(7): 2800-2809.

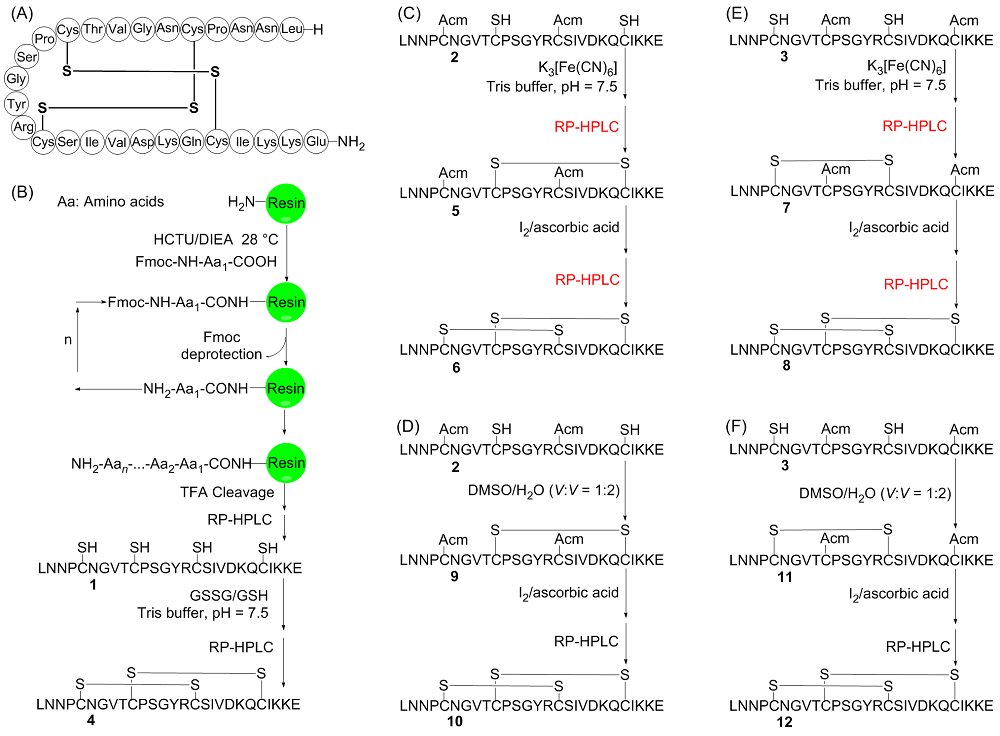
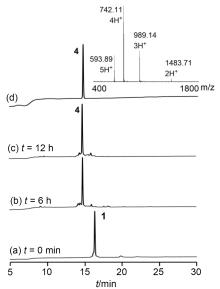



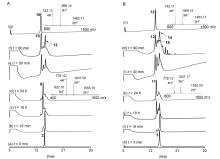
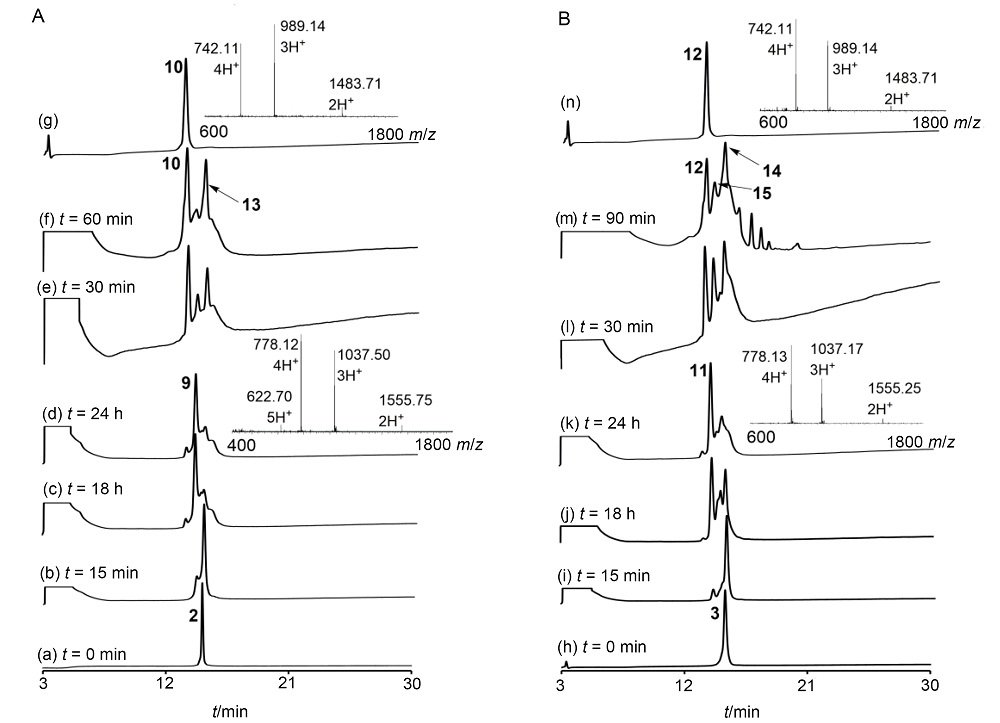




| [10] |
(f) Wu, Y; Wu, X.; Zhangsun, D.; Luo, S. Biotechnol. Bull. 2013, 7,184 (in Chinese).
|
|
( 吴勇, 吴潇洒, 长孙东亭, 罗素兰, 生物技术通报, 2013, 7,184.)
|
|
| [11] |
(a) Cuthbertson, A.; Indrevoll, B. Org. Lett. 2003, 5,2955.
doi: 10.1021/ol035105w |
|
(b) Naraga,A. M.B.; Belleza,O. J.V.; Villaraza,A. J.L. RSC Adv. 2018, 8,36579.
doi: 10.1039/C8RA03706J |
|
|
(c) Khemtémourian, L.; Desbenoit, N; Mahesh, P.; Chatterjee, S.; Deschemin,J. -C.; Vaulont, S.; Tomas, A.; Sari,M. -A.; Artaud, I. Protein Pept. Lett. 2012, 19,219.
doi: 10.2174/092986612799080167 |
|
| [12] |
(a) Cui,H. -K.; Guo, Y.; He, Y.; Wang,F. -L.; Chang,H. -N.; Wang,Y. -J.; Wu,F. -M.; Tian,C. -L.; Liu, L. Angew. Chem.,Int. Ed. 2013, 52,9558.
doi: 10.1002/anie.v52.36 |
|
(b) Guo, Y.; Sun,D. -M.; Wang,F. -L.; He, Y.; Liu, L.; Tian,C. -L. Angew. Chem.,Int. Ed. 2015, 54,14276.
doi: 10.1002/anie.201500699 |
|
|
(c) Xu, Y.; Wang, T.; Guan,C. -J.; Li,Y. -M.; Liu, L.; Shi, J.; Bierer, D. Tetrahedron Lett. 2017, 58,1677.
doi: 10.1016/j.tetlet.2017.03.024 |
|
|
(d) Wang, T.; Fan, J.; Chen,X. -X.; Zhao, R.; Xu, Y.; Bierer, D.; Liu, L.; Li,Y. -M.; Shi, J.; Fang,G. -M. Org. Lett. 2018, 20,6074.
doi: 10.1021/acs.orglett.8b02459 |
|
|
(e) Qi,Y. -K.; Qu, Q.; Bierer, D.; Liu, L. Chem.-Asian J. 2020, 15,2793.
doi: 10.1002/asia.v15.18 |
|
|
(f) Qu, Q.; Gao, S.; Wu, F.; Zhang,M. -G.; Li, Y.; Zhang,L. -H.; Bierer, D.; Tian,C. -L.; Zheng,J. -S.; Liu, L. Angew. Chem.,Int. Ed. 2020, 59,6037.
doi: 10.1002/anie.v59.15 |
|
|
(g) Chen, J.; Sun, S.; Zhao, R.; Xi,C. -P.; Qiu, W.; Wang, N.; Wang, Y.; Bierer, D.; Shi, J.; Li,Y. -M. ChemistrySelect 2020, 5,1359.
doi: 10.1002/slct.v5.4 |
|
|
(h) Guo, Y.; Liu, C.; Song, H.; Wang,F. -L.; Zou, Y.; Wu,Q. -Y.; Hu,H. -G. RSC Adv. 2017, 7,2110.
doi: 10.1039/C6RA26617G |
|
|
(i) Huang,D. -L.; Bai,J. -S.; Wu, M.; Wang, X.; Riedl, B.; Pook, E.; Alt, C.; Erny, M.; Li,Y. -M.; Bierer, D.; Shi, J.; Fang,G. -M. Chem. Commun. 2019, 55,2821.
doi: 10.1039/C9CC00328B |
|
|
(j) Wang,F. -L.; Guo, Y.; Li,S. -J.; Guo,Q. -X.; Shi, J.; Li,Y. -M. Org. Biomol. Chem. 2015, 13,6286.
doi: 10.1039/C5OB00741K |
|
|
(k) Wang, T.; Kong,Y. -F.; Xu, Y.; Fan, J.; Xu,H. -J.; Bierer, D.; Wang, J.; Shi, J.; Li,Y. -M. Tetrahedron Lett. 2017, 58,3970.
doi: 10.1016/j.tetlet.2017.09.006 |
|
| [1] |
(a) Daly,N. L.; Craik,D. J. Curr. Opin. Chem. Biol. 2011, 15,362.
doi: 10.1016/j.cbpa.2011.02.008 |
|
(b) Silva,P. M.; Gonçalves, S.; Santos,N. C. Front. Microbiol. 2014, 5,97.
|
|
|
(c) Tanemura, Y.; Mochizuki, Y.; Kumachi, S.; Nemoto, N. Biology 2015, 4,161.
doi: 10.3390/biology4010161 |
|
|
(d) Wu, Q.; Liu, Z.; Fu, C.; Lin, Y.; Dai, Q. Chin. J. Org. Chem. 2010, 30,1517 (in Chinese).
doi: 10.1002/cjoc.v30.7 |
|
|
( 吴巧玲, 刘珠果, 付超, 林原斌, 戴秋云, 有机化学, 2010, 30,1517.)
|
|
|
(e) Chi, Y-S.; Zhang,H. -B.; Ni,S. -J.; Huang,W. -L. Chin. J. Org. Chem. 2008, 28,416 (in Chinese).
|
|
|
( 迟玉石, 张惠斌, 倪帅健, 黄文龙, 有机化学, 2008, 28,416.)
|
|
| [2] |
(a) Góngora-Benítez, M.; Tulla-Puche, J.; Albericio, F. Chem. Rev. 2014, 114,901.
doi: 10.1021/cr400031z |
|
(b) Cemazar, M.; Kwon, S.; Mahatmanto, T.; Ravipati,A. S.; Craik,D. J. Curr. Top. Med. Chem. 2012, 12,1534.
doi: 10.2174/156802612802652484 |
|
|
(c) Akondi,K. B.; Muttenthaler, M.; Dutertre, S.; Kaas, Q.; Craik,D. J.; Lewis,R. J.; Alewood,P. F. Chem. Rev. 2014, 114,5815.
doi: 10.1021/cr400401e |
|
|
(d) Sun,S. -S.; Chen, J.; Zhao, R.; Bierer, D.; Wang, J.; Fang,G. -M.; Li,Y. -M. Tetrahedron Lett. 2019, 60,1197.
|
|
|
(e) Pan, X.; Li, Z.; Huang, X.; Huang, G.; Gao, S.; Shen, H.; Liu, L.; Lei, J.; Yan, N. Science 2019, 363,1309.
doi: 10.1126/science.aaw2999 |
|
|
(f) Lin King, J.V.; Emrick, J.J.; Kelly, M.J.S.; Herzig, V.; King, G.F.; Medzihradszky, K.F.; Julius, D. Cell 2019, 178,1362.
doi: 10.1016/j.cell.2019.07.014 |
|
|
(g) Guo,X. -Q.; Liang, J.; Li, Y.; Zhang, Y.; Huang, D.; Tian, C. Chin. Chem. Lett. 2018, 29,1139.
doi: 10.1016/j.cclet.2018.05.005 |
|
|
(h) Sun, D.; Liu, S.; Li, S.; Zhang, M.; Yang, F.; Wen, M.; Shi, P.; Wang, T.; Pan, M.; Chang, S.; Zhang, X.; Zhang, L.; Tian, C.; Liu, L. eLife 2020, 9,e57096.
doi: 10.7554/eLife.57096 |
|
|
(i) Zhu, W.; Hou, F.; Fang, J.; Bahrani Fard,M. R.; Liu, Y.; Ren, S.; Wu, S.; Qi, Y.; Sui, S.; Read,A. T.; Sherwood,J. M.; Zou, W.; Yu, H.; Zhang, J.; Overby,D. R.; Wang, N.; Ethier,C. R.; Wang, K. iScience 2021, 24,102042.
doi: 10.1016/j.isci.2021.102042 |
|
|
(j) Song, H.; Liu, C.; Wu, Y.; Hu, H.; Yan, F. Acta Chim. Sinica 2018, 76,95 (in Chinese).
doi: 10.6023/A17100473 |
|
| [13] |
(a) Zheng,J. -S.; Tang, S.; Qi,Y. -K.; Wang,Z. -P.; Liu, L. Nat. Protoc. 2013, 8,2483.
doi: 10.1038/nprot.2013.152 |
|
(b) Qi,Y. -K.; Tang, S.; Huang,Y. -C.; Pan, M.; Zheng,J. -S.; Liu, L. Org. Biomol. Chem. 2016, 14,4194.
doi: 10.1039/C6OB00450D |
|
|
(c) Qi,Y. -K.; He,Q. -Q.; Ai,H. -S.; Guo, J.; Li,J. -B. Chem. Commun. 2017, 53,4148.
doi: 10.1039/C7CC01721A |
|
|
(d) Qi,Y. -K.; He,Q. -Q.; Ai,H. -S.; Li,J. -B.; Zheng,J. -S. Synlett 2017, 28,1907.
doi: 10.1055/s-0036-1590794 |
|
|
(e) Li,J. -B.; Qi,Y. -K.; He,Q. -Q.; Ai,H. -S.; Liu,S. -L.; Wang,J. -X.; Zheng,J. -S.; Liu, L.; Tian, C. Cell Res. 2018, 28,257.
doi: 10.1038/cr.2017.157 |
|
|
(f) Qi,Y. -K.; Ai,H. -S.; Li,Y. -M.; Yan, B. Front. Chem. 2018, 6,19.
doi: 10.3389/fchem.2018.00019 |
|
| [14] |
(a) Li, Z.; Zhang, B.; Zuo, C.; Liu, L. Chin. J. Org. Chem. 2018, 38,2412 (in Chinese).
doi: 10.6023/cjoc201804014 |
|
( 黎子琛, 张宝昌, 左冲, 刘磊, 有机化学, 2018, 38,2412.)
|
|
|
(b) Qi,Y. -K.; Si,Y. -Y.; Du,S. -S.; Liang, J.; Wang,K. W.; Zheng,J. -S. Sci. China: Chem. 2019, 62,299.
doi: 10.1007/s11426-018-9401-8 |
|
|
(c) Zhang, B.; Deng, Q.; Zuo, C.; Yan, B.; Zuo, C.; Cao,X. -X.; Zhu,T. F.; Zheng,J. -S.; Liu, L. Angew. Chem.,Int. Ed. 2019, 58,12231.
doi: 10.1002/anie.v58.35 |
|
|
(d) Zuo, C.; Shi,W. -W.; Chen,X. -X.; Glatz, M.; Riedl, B.; Flamme, I.; Pook, E.; Wang, J.; Fang,G. -M.; Bierer, D.; Liu, L. Sci. China: Chem. 2019, 62,1371.
doi: 10.1007/s11426-019-9513-2 |
|
|
(e) Zhang, B.; Li, Y.; Shi, W.; Wang, T.; Zhang, F.; Liu, L. Chem. Res. Chin. Univ. 2020, 36,733.
doi: 10.1007/s40242-020-0150-y |
|
|
(f) Tang, S.; Zheng, J.; Yang, K.; Liu, L. Acta Chim. Sinica 2012, 70,1471 (in Chinese).
doi: 10.6023/A12040166 |
|
|
( 唐姗, 郑基深, 杨可, 刘磊, 化学学报, 2012, 70,1471.)
|
|
|
(g) Fang,G. -M.; Li,Y. -M.; Shen, F.; Huang,Y. -C.; Li,J. -B.; Lin, Y.; Cui,H. -K.; Liu, L. Angew. Chem.,Int. Ed. 2011, 50,7645.
doi: 10.1002/anie.201100996 |
|
| [2] |
( 宋慧, 刘超, 吴仪君, 胡宏岗, 阎芳, 化学学报, 2018, 76,95.)
|
| [3] |
(a) Xu, X.; Xu, Q.; Chen, F.; Shi, J.; Liu, Y.; Chu, Y.; Wan, S.; Jiang, T.; Yu, R. RSC Adv. 2019, 9,668.
doi: 10.1039/C8RA06103C |
|
(b) Zheng, Y.; Zhai, L.; Zhao, Y.; Wu, C. J. Am. Chem. Soc. 2015, 137,15094.
doi: 10.1021/jacs.5b10779 |
|
|
(c) Chen, C.; Gao, S.; Qu, Q.; Mi, P.; Tao, A.; Li,Y. -M. Chin. Chem. Lett. 2018, 29,1135.
doi: 10.1016/j.cclet.2018.01.005 |
|
|
(d) Liu, J.; Dong, S. Chin. Chem. Lett. 2018, 29,1131.
doi: 10.1016/j.cclet.2018.05.014 |
|
|
(e) Zhao, R.; Shi, P.; Chen, J.; Sun, S.; Chen, J.; Cui, J.; Wu, F.; Fang, G.; Tian, C.; Shi, J.; Bierer, D.; Liu, L.; Li,Y. -M. Chem. Sci. 2020, 11,7927.
doi: 10.1039/D0SC02374D |
|
|
(f) Ge, W.; Chen, J.; Zhang, Y.; Zong, L.; Zhang, M.; Dong, J. Chin. J. Org. Chem. 2017, 37,2409 (in Chinese).
doi: 10.6023/cjoc201704020 |
|
|
( 葛巍巍, 陈静, 张也, 宗良, 张鸣, 董俊军, 有机化学, 2017, 37,2409.)
|
|
| [4] |
Yang, S.; Yang, F.; Wei, N.; Hong, J.; Li, B.; Luo, L.; Rong, M.; Yarov-Yarovoy, V.; Zheng, J.; Wang, K.; Lai, R. Nat. Commun. 2015, 6,8297.
doi: 10.1038/ncomms9297 |
| [5] |
(a) Chu, Y.; Qiu, P.; Yu, R. Toxins 2020, 12,230.
doi: 10.3390/toxins12040230 |
|
(b) Zhu, A.; Aierken, A.; Yao, Z.; Vu, S.; Tian, Y.; Zheng, J.; Yang, S.; Yang, F. Toxicon 2020, 178,41.
doi: 10.1016/j.toxicon.2020.02.016 |
|
|
(c) Du, G.; Tian, Y.; Yao, Z.; Vu, S.; Zheng, J.; Chai, L.; Wang, K.; Yang, S. J. Biol. Chem. 2020, 295,9641.
doi: 10.1074/jbc.RA120.013037 |
|
|
(d) Luo, L.; Wang, Y.; Li, B.; Xu, L.; Kamau,P. M.; Zheng, J.; Yang, F.; Yang, S.; Lai, R. Nat. Commun. 2019, 10,2134.
doi: 10.1038/s41467-019-09965-6 |
|
|
(e) Ombati, R.; Luo, L.; Yang, S.; Lai, R. Toxicon 2018, 154,60.
doi: 10.1016/j.toxicon.2018.09.008 |
|
|
(f) Yu, R.; Liu, H.; Wang, B.; Harvey,P. J.; Wei, N.; Chu, Y. RSC Adv. 2020, 10,2141.
doi: 10.1039/C9RA08829F |
|
| [6] |
Lyu,H. -N.; Wei,N. -N.; Tu,P. -F.; Wang, K.; Jiang, Y. Nat. Prod. Res. 2020, 34,1068.
doi: 10.1080/14786419.2018.1548455 |
| [7] |
(a) Karas,J. A.; Patil,N. A.; Tailhades, J.; Sani,M. -A.; Scanlon,D. B.; Forbes,B. E.; Gardiner, J.; Separovic, F.; Wade,J. D.; Hossain,M. A. Angew. Chem.,Int. Ed. 2016, 55,14743.
doi: 10.1002/anie.v55.47 |
|
(b) Tang, S.; Si,Y. -Y.; Wang,Z. -P.; Mei,K. -R.; Chen, X.; Cheng,J. -Y.; Zheng,J. -S.; Liu, L. Angew. Chem.,Int. Ed. 2015, 54,5713.
doi: 10.1002/anie.201500051 |
|
| [8] |
(a) Qu, Q.; Gao, S.; Li,Y. -M. J. Pept. Sci. 2018, 24,e3112.
doi: 10.1002/psc.v24.8-9 |
|
(b) Muttenthaler, M.; Nevin,S. T.; Grishin,A. A.; Ngo,S. T.; Choy,P. T.; Daly,N. L.; Hu,S. -H.; Armishaw,C. J.; Wang,C. -I.A.; Lewis,R. J.; Martin,J. L.; Noakes,P. G.; Craik,D. J.; Adams,D. J.; Alewood,P. F. J. Am. Chem. Soc. 2010, 132,3514.
doi: 10.1021/ja910602h |
|
|
(c) Lan, H.; Wu, K.; Zheng, Y.; Pan, M.; Huang,Y. -C.; Gao, S.; Zheng,Q. -Y.; Zheng,J. -S.; Li,Y. -M.; Xiao, B.; Liu, L. J. Pept. Sci. 2016, 22,320.
doi: 10.1002/psc.2868 |
|
|
(d) Chang,H. -N.; Liu,B. -Y.; Qi,Y. -K.; Zhou, Y.; Chen,Y. -P.; Pan,K. -M.; Li,W. -W.; Zhou,X. -M.; Ma,W. -W.; Fu,C. -Y.; Qi,Y. -M.; Liu, L.; Gao,Y. -F. Angew. Chem.,Int. Ed. 2015, 54,11760.
doi: 10.1002/anie.201506225 |
|
|
(e) Zhou, X.; Zuo, C.; Li, W.; Shi, W.; Zhou, X.; Wang, H.; Chen, S.; Du, J.; Chen, G.; Zhai, W.; Zhao, W.; Wu, Y.; Qi, Y.; Liu, L.; Gao, Y. Angew. Chem.,Int. Ed. 2020, 59,15114.
doi: 10.1002/anie.v59.35 |
|
| [9] |
(a) Mochizuki, M.; Tsuda, S.; Tanimura, K.; Nishiuchi, Y. Org. Lett. 2015, 17,2202.
doi: 10.1021/acs.orglett.5b00786 |
|
(b) Jordan,J. B.; Poppe, L.; Haniu, M.; Arvedson, T.; Syed, R.; Li, V.; Kohno, H.; Kim, H.; Schnier,P. D.; Harvey,T. S.; Miranda,L. P.; Cheetham, J.; Sasu,B. J. J. Biol. Chem. 2009, 284,24155.
doi: 10.1074/jbc.M109.017764 |
|
| [10] |
(a) Dowell, C.; Olivera,B. M.; Garrett,J. E.; Staheli,S. T.; Watkins, M.; Kuryatov, A.; Yoshikami, D.; Lindstrom,J. M.; McIntosh,J. M. J. Neurosci. 2003, 23,8445.
doi: 10.1523/JNEUROSCI.23-24-08445.2003 |
|
(b) Luo, S.; Akondi,K. B.; Zhangsun, D.; Wu, Y.; Zhu, X.; Hu, Y.; Christensen, S.; Dowell, C.; Daly,N. L.; Craik,D. J.; Wang,C. -I.A.; Lewis,R. J.; Alewood,P. F.; McIntosh,J. M. J. Biol. Chem. 2010, 285,12355.
doi: 10.1074/jbc.M109.079012 |
|
|
(c) Luo, S.; Christensen, S.; Zhangsun, D.; Wu, Y.; Hu, Y.; Zhu, X.; Chhabra, S.; Norton,R. S.; McIntosh,J. M. PLoS One 2013, 8,e54648.
doi: 10.1371/journal.pone.0054648 |
|
|
(d) Shi, J.; So,L. -Y.; Chen, F.; Liang, J.; Chow,H. -Y.; Wong,K. -Y.; Wan, S.; Jiang, T.; Yu, R. J. Pept. Sci. 2018, 24,e3087.
doi: 10.1002/psc.v24.6 |
|
|
(e) Wu, Y.; Wu, X.; Yu, J.; Zhu, X.; Zhangsun, D.; Luo, S. Molecules 2014, 19,966.
doi: 10.3390/molecules19010966 |
| [1] | Yannan Ma, Ya'ni Liu, Jinyan Wang, Xitong Chen, Hao Yin, Qiaona Chi, Shixi Jia, Shanshan Du, Yunkun Qi, Kewei Wang. DIC/Oxyma Based Efficient Synthesis and Activity Evaluation of Spider Peptide Toxin GsMTx4 [J]. Chinese Journal of Organic Chemistry, 2022, 42(2): 498-506. |
| [2] | LI Xue-Qiang, WEI Chuan-Wan, LIU Xiao-Fang, LIU You-Nian. Synthesis of Ferrocenoyl-peptide and Its Inhibition for β-Amyloid Peptide [J]. Chin. J. Org. Chem., 2010, 30(10): 1492-1496. |
| [3] | WU Qiao-Ling, LIU Zhu-Guo, FU Chao, LIN Yuan-Bin, DAI Qiu-Yun. A Mimetic Peptide with Two Pairs of Concentrated Disulfide Bridges [J]. Chin. J. Org. Chem., 2010, 30(10): 1517-1520. |
| [4] | YANG Ming,WANG Wei-Guo,LI Pei-Hua,ZHANG Jun,SHEN Shu-Bao*. Solid Phase Peptide Coupling and Its Kinetics under Microwave Irradiation [J]. Chin. J. Org. Chem., 2008, 28(05): 851-856. |
| [5] | CHI Yu-Shi,ZHANG Hui-Bin*,NI Shuai-Jian,HUANG Wen-Long. Solid Phase Synthesis of Cyclopeptides of Oxytocin and Lysipressin under Microwave Irradiation [J]. Chin. J. Org. Chem., 2008, 28(03): 416-421. |
| [6] | HUANG Xiao-Yi, WANG Tao, XIA Chuan-Qin, YU Xiao-Qi, XIE Ru-Gang. Synthesis of Novel Disulfide Bond-Bearing Cyclopeptides [J]. Chinese Journal of Organic Chemistry, 2004, 24(12): 1629-1632. |
| [7] | Jiang Hui;Zhong Mingnai;Chen Jisheng;Miao Zhenwei. The advances in the synthesis of multiple disulfide bond-containing peptides [J]. Chin. J. Org. Chem., 1999, 19(3): 214-223. |
| [8] | Wang Liangyou;Pan Heping;Chen Zhengying. Methods of disulfide formation in peptide synthesis [J]. Chin. J. Org. Chem., 1998, 18(6): 576-580. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||