Chinese Journal of Organic Chemistry ›› 2021, Vol. 41 ›› Issue (9): 3668-3674.DOI: 10.6023/cjoc202104021 Previous Articles Next Articles
Special Issue: 镍催化有机反应虚拟合辑
ARTICLES
收稿日期:2021-04-10
修回日期:2021-06-07
发布日期:2021-06-17
通讯作者:
周维友
基金资助:
Junfeng Qian, Xiaoting Tian, Zhong Wu, Jie Yao, Hui Wang, Weiyou Zhou( )
)
Received:2021-04-10
Revised:2021-06-07
Published:2021-06-17
Contact:
Weiyou Zhou
Supported by:Share
Junfeng Qian, Xiaoting Tian, Zhong Wu, Jie Yao, Hui Wang, Weiyou Zhou. Efficient Oxidative Coupling of Isochroman with Primary Arylamines Catalyzed by Heterogeneous Ni-Containing Layered Double Oxide[J]. Chinese Journal of Organic Chemistry, 2021, 41(9): 3668-3674.
| Entry | Catalyst | Solvent | Oxidant | 2a/1 | Temp./℃ | Conv.b/% | Sel.b/% |
|---|---|---|---|---|---|---|---|
| 1 | Ni3Ga-LDH | Toluene | TBHP | 1 | 80 | 42 | 50 |
| 2 | Ni3Ga-LDO | Toluene | TBHP | 1 | 80 | 65 | 58 |
| 3 | Ni3Al-LDO | Toluene | TBHP | 1 | 80 | 60 | 34 |
| 4 | Ni3In-LDO | Toluene | TBHP | 1 | 80 | 44 | 25 |
| 5 | Ni3Ga-LDO | Acetonitrile | TBHP | 1 | 80 | 80 | 28 |
| 6 | Ni3Ga-LDO | Dioxane | TBHP | 1 | 80 | 79 | 37 |
| 7 | Ni3Ga-LDO | Methanol | TBHP | 1 | 80 | 60 | 28 |
| 8 | Ni3Ga-LDO | H2O | TBHP | 1 | 80 | Trace | — |
| 9 | Ni3Ga-LDO | DMSO | TBHP | 1 | 80 | Trace | — |
| 10 | Ni3Ga-LDO | 1,1,2,2-Tetrachloroethane | TBHP | 1 | 80 | 73 | 60 |
| 11 | Ni3Ga-LDO | 1,1,2,2-Tetrachloroethane | DTBP | 1 | 80 | Trace | — |
| 12 | Ni3Ga-LDO | 1,1,2,2-Tetrachloroethane | Na2S2O8 | 1 | 80 | Trace | — |
| 13 | Ni3Ga-LDO | 1,1,2,2-Tetrachloroethane | O2 | 1 | 80 | Trace | — |
| 14 | Ni3Ga-LDO | 1,1,2,2-Tetrachloroethane | TBHP | 2 | 80 | 77 | 70 |
| 15 | Ni3Ga-LDO | 1,1,2,2-Tetrachloroethane | TBHP | 4 | 80 | 82 | 48 |
| 16 | Ni3Ga-LDO | 1,1,2,2-Tetrachloroethane | TBHP | 2 | 60 | 60 | 40 |
| 17 | Ni3Ga-LDO | 1,1,2,2-Tetrachloroethane | TBHP | 2 | 100 | 72 | 64 |
| 18c | Ni3Ga-LDO | 1,1,2,2-Tetrachloroethane | TBHP | 2 | 80 | 82 | 75 |
| 19d | Ni3Ga-LDO | 1,1,2,2-Tetrachloroethane | TBHP | 2 | 80 | 94 | 34 |
| 20c,e | Ni3Ga-LDO | 1,1,2,2-Tetrachloroethane | TBHP | 2 | 80 | 86 | 77 |
| 21c,f | Ni3Ga-LDO | 1,1,2,2-Tetrachloroethane | TBHP | 2 | 80 | 92 | 70 |
| Entry | Catalyst | Solvent | Oxidant | 2a/1 | Temp./℃ | Conv.b/% | Sel.b/% |
|---|---|---|---|---|---|---|---|
| 1 | Ni3Ga-LDH | Toluene | TBHP | 1 | 80 | 42 | 50 |
| 2 | Ni3Ga-LDO | Toluene | TBHP | 1 | 80 | 65 | 58 |
| 3 | Ni3Al-LDO | Toluene | TBHP | 1 | 80 | 60 | 34 |
| 4 | Ni3In-LDO | Toluene | TBHP | 1 | 80 | 44 | 25 |
| 5 | Ni3Ga-LDO | Acetonitrile | TBHP | 1 | 80 | 80 | 28 |
| 6 | Ni3Ga-LDO | Dioxane | TBHP | 1 | 80 | 79 | 37 |
| 7 | Ni3Ga-LDO | Methanol | TBHP | 1 | 80 | 60 | 28 |
| 8 | Ni3Ga-LDO | H2O | TBHP | 1 | 80 | Trace | — |
| 9 | Ni3Ga-LDO | DMSO | TBHP | 1 | 80 | Trace | — |
| 10 | Ni3Ga-LDO | 1,1,2,2-Tetrachloroethane | TBHP | 1 | 80 | 73 | 60 |
| 11 | Ni3Ga-LDO | 1,1,2,2-Tetrachloroethane | DTBP | 1 | 80 | Trace | — |
| 12 | Ni3Ga-LDO | 1,1,2,2-Tetrachloroethane | Na2S2O8 | 1 | 80 | Trace | — |
| 13 | Ni3Ga-LDO | 1,1,2,2-Tetrachloroethane | O2 | 1 | 80 | Trace | — |
| 14 | Ni3Ga-LDO | 1,1,2,2-Tetrachloroethane | TBHP | 2 | 80 | 77 | 70 |
| 15 | Ni3Ga-LDO | 1,1,2,2-Tetrachloroethane | TBHP | 4 | 80 | 82 | 48 |
| 16 | Ni3Ga-LDO | 1,1,2,2-Tetrachloroethane | TBHP | 2 | 60 | 60 | 40 |
| 17 | Ni3Ga-LDO | 1,1,2,2-Tetrachloroethane | TBHP | 2 | 100 | 72 | 64 |
| 18c | Ni3Ga-LDO | 1,1,2,2-Tetrachloroethane | TBHP | 2 | 80 | 82 | 75 |
| 19d | Ni3Ga-LDO | 1,1,2,2-Tetrachloroethane | TBHP | 2 | 80 | 94 | 34 |
| 20c,e | Ni3Ga-LDO | 1,1,2,2-Tetrachloroethane | TBHP | 2 | 80 | 86 | 77 |
| 21c,f | Ni3Ga-LDO | 1,1,2,2-Tetrachloroethane | TBHP | 2 | 80 | 92 | 70 |
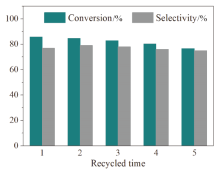
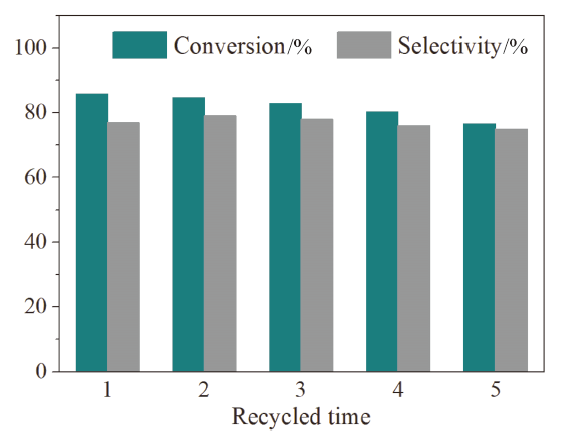
| Entry | Catalyst | Additive | Conv.b/% | Sel.b/% |
|---|---|---|---|---|
| 1 | Ni3Ga-LDO | — | 86 | 77 |
| 2 | — | — | 52 | 38 |
| 3c | Ni3Ga-LDO (air) | — | 79 | 13 |
| 4 | Mg3Ga-LDO | — | Trace | — |
| 5d | Ni2(AcO)2 | — | 76 | 39 |
| 6 | Ni3Ga-LDO | BHTe | 13 | 63 |
| 7 | Ni3Ga-LDO | TEMPOe | 82 | 52 |
| Entry | Catalyst | Additive | Conv.b/% | Sel.b/% |
|---|---|---|---|---|
| 1 | Ni3Ga-LDO | — | 86 | 77 |
| 2 | — | — | 52 | 38 |
| 3c | Ni3Ga-LDO (air) | — | 79 | 13 |
| 4 | Mg3Ga-LDO | — | Trace | — |
| 5d | Ni2(AcO)2 | — | 76 | 39 |
| 6 | Ni3Ga-LDO | BHTe | 13 | 63 |
| 7 | Ni3Ga-LDO | TEMPOe | 82 | 52 |
| [1] |
(a) Xu, Z.; Zheng, Y.; Wang, Z.; Shao, X.; Wang, Y. Chem. Commun. 2019, 55, 15089.
doi: 10.1039/C9CC08622F |
|
(b) Nasresfahani, Z.; Kassaee, M. Z. Appl. Organomet. Chem. 2021, 35(1), e6032.
|
|
|
(c) Luo, N. H.; Zhong, Y. H.; Wen, H. L.; Luo, R. ACS Omega 2020, 5, 27723.
doi: 10.1021/acsomega.0c04192 |
|
| [2] |
(a) Ghorai, M. K.; Nanaji, Y. J. Org. Chem. 2013, 78, 3867.
doi: 10.1021/jo400287a pmid: 23548056 |
|
(b) Larsen, A. F.; Ulven, T. Chem. Commun. 2014, 50, 4997.
doi: 10.1039/C3CC48642G pmid: 23548056 |
|
|
(c) Xie, J. W.; Wang, X. C.; Wu, F. T.; Zhang, J. Chin. J. Org. Chem. 2019, 39, 3026. (in Chinese).
doi: 10.6023/cjoc201907051 pmid: 23548056 |
|
|
( 谢建伟, 汪小创, 吴丰田, 张洁, 有机化学, 2019, 39, 3026.)
pmid: 23548056 |
|
|
(d) Yao, D. D.; Zhang, J. L.; Xu, L. Chin. J. Org. Chem. 2020, 40, 1673. (in Chinese).
doi: 10.6023/cjoc201912038 pmid: 23548056 |
|
|
( 姚丹丹, 张金利, 徐亮, 有机化学, 2020, 40, 1673.)
pmid: 23548056 |
|
| [3] |
(a) Irrgang, T.; Kempe, R. Chem. Rev. 2020, 120, 9583.
doi: 10.1021/acs.chemrev.0c00248 |
|
(b) Montes-Andrés, H.; Leo, P.; Muñoz, A.; Rodríguez-Diéguez, A.; Orcajo, G.; Choquesillo-Lazarte, D.; Martos, C.; Martínez, F.; Botas, J. A.; Calleja, G. Inorg. Chem. 2020, 59, 15733.
doi: 10.1021/acs.inorgchem.0c02127 |
|
|
(c) Bhoge, B. A.; Mala, P.; Kurian, J. S.; Srinivasan, V.; Saraogi, I. Chem. Commun. 2020, 56, 13832.
doi: 10.1039/D0CC05492E |
|
|
(d) Ravi, K.; Advani, J. H.; Bankar, B. D.; Singh, A. S.; Biradar, A. V. New J. Chem. 2020, 44, 18714.
doi: 10.1039/D0NJ04673F |
|
|
(e) Petersen, A. R.; Lauridsen, J. M. V.; Lee, J. W. Eur. J. Org. Chem. 2020, 47, 7368.
|
|
|
(f) Yan, F.; Cai, S.; Wen, W.; Wen, W.; Li, B. J.; Wang, L. S.; Zhu, L. Chin. J. Org. Chem. 2020, 40, 1874. (in Chinese).
doi: 10.6023/cjoc201912031 |
|
|
( 严沣, 蔡爽, 闻武, 文蔚, 李博解, 汪连生, 朱磊, 有机化学, 2020, 40, 1874.)
|
|
| [4] |
(a) Aneeja, T.; Neetha, M.; Afsinaa, C. M. A.; Anilkumar, G. RSC Adv. 2020, 10, 34429.
doi: 10.1039/D0RA06518H pmid: 23298175 |
|
(b) Anastas, P.; Eghbali, N. Chem. Soc. Rev. 2010, 39, 301.
doi: 10.1039/b918763b pmid: 23298175 |
|
|
(c) Wang, G.; Wang, J. T.; Zhao, B.; Chen, H.; Zhang, P. K.; Han, S. L. Tetrahedron Lett. 2020, 61, 151426.
doi: 10.1016/j.tetlet.2019.151426 pmid: 23298175 |
|
|
(d) Zhang, X. L.; Pan, G. F.; Zhu, X. Q.; Guo, R. L.; Gao, Y. R.; Wang, Y. Q. Org. Lett. 2019, 21, 2731.
doi: 10.1021/acs.orglett.9b00695 pmid: 23298175 |
|
|
(e) Baslé, O.; Li, C. J. Chem. Commun. 2009, 27, 4124.
pmid: 23298175 |
|
|
(f) Boess, E.; Schmitz, C.; Klussmann, M. J. Am. Chem. Soc. 2012, 134, 5317.
doi: 10.1021/ja211697s pmid: 23298175 |
|
|
(g) Wusiman, A.; Hudabaierdi, R. Tetrahedron Lett. 2019, 60, 681.
doi: 10.1016/j.tetlet.2019.01.058 pmid: 23298175 |
|
|
(h) Liu, Y. X.; Wang, C.; Xue, D.; Xiao, M.; Li, C. Q.; Xiao, J. L. Chem.-Eur. J. 2017, 23, 3051.
pmid: 23298175 |
|
|
(i) Brzozowski, M.; Forni, J. A.; Savage, G. P.; Polyzos, A. Chem. Commun. 2015, 51, 334.
doi: 10.1039/C4CC07913B pmid: 23298175 |
|
|
(j) Sun, Y. M.; Ding, Q. F.; Yu, Y.; He, Y. D.; Huang, F. Chin. J. Org. Chem. 2019, 39, 3363. (in Chinese).
doi: 10.6023/cjoc201906026 pmid: 23298175 |
|
|
( 孙义明, 丁奇峰, 于杨, 何益得, 黄菲, 有机化学, 2019, 39, 3363.)
pmid: 23298175 |
|
|
(k) Rana, P.; Gaur, R.; Gupta, R.; Arora, G.; Jayashree, A.; Sharama, R. K. Chem. Commun. 2019, 55, 7402.
doi: 10.1039/C9CC02386K pmid: 23298175 |
|
|
(l) Xie, J.; Li, H. M.; Zhou, J. C.; Cheng, Y. X.; Zhu, C. J. Angew. Chem. Int. Ed. 2012, 51, 1252.
doi: 10.1002/anie.201107605 pmid: 23298175 |
|
|
(m) Ratnikov, M. O.; Doyle, M. P.; J. Am. Chem. Soc. 2013, 135, 1549.
doi: 10.1021/ja3113559 pmid: 23298175 |
|
|
(n) Patil, M. R.; Dedhia, N. P.; Kapdi, A. R.; Kumar, A. V. J. Org. Chem. 2018, 83, 4477.
doi: 10.1021/acs.joc.8b00203 pmid: 23298175 |
|
|
(o) Kumar, R. A.; Saidulu, G.; Prasad, K. R.; Kumar, G. S.; Sridhar, B.; Reddy, K. R. Adv. Synth. Catal. 2012, 354, 2985.
doi: 10.1002/adsc.v354.16 pmid: 23298175 |
|
|
(p) Cao, M.; Mao, Y.; Huang, J. C.; Ma, Y. D.; Liu, L. Tetrahedron Lett. 2019, 60, 1075.
doi: 10.1016/j.tetlet.2019.03.032 pmid: 23298175 |
|
| [5] |
(a) Martin, P.; Consroe, P. Science 1976, 194, 965.
pmid: 982057 |
|
(b) Groot, M. J. D.; Alex, A. A.; Jones, B. C. J. Med. Chem. 2002, 45, 1983.
doi: 10.1021/jm0110791 pmid: 982057 |
|
|
(c) Yamaori, S.; Kushihara, M.; Yamamoto, I.; Watanabe, Y. Biochem. Pharmacol. 2010, 79, 1691.
doi: 10.1016/j.bcp.2010.01.028 pmid: 982057 |
|
| [6] |
(a) Lehmann, F.; Pettersen, A.; Currier, E. A.; Sherbukhin, V.; Olsson, R.; Hacksell, U., Luthman, K. J. Med. Chem. 2006, 49, 2232.
doi: 10.1021/jm051121i |
|
(b) Maier, C. A.; Wünsch, B. J. Med. Chem. 2002, 45, 438.
doi: 10.1021/jm010992z |
|
| [7] |
Feng, J.; Lv, M. F.; Lu, G. P.; Cai, C. Org. Chem. Front. 2015, 2, 60.
doi: 10.1039/C4QO00293H |
| [8] |
Chen, D.; Pan, F.; Gao, J.; Yang, J. Synlett 2013, 24, 2085.
doi: 10.1055/s-00000083 |
| [9] |
(a) Zhou, W. Y.; Lu, W. M. Z.; Wang, H.; Xia, Z. Z.; Zhai, S. Y.; Zhang, Z.; Ma, Y. J.; He, M. Y.; Chen, Q. Appl. Catal. A, Gen. 2020, 604, 117771.
doi: 10.1016/j.apcata.2020.117771 |
|
(b) Sun, Z. H.; Xia, Z. Z.; Wang, A. W.; Wang, H.; Zhang, Z.; Zhou, W. Y.; Qian, J. F.; He, M. Y. Tetrahedron Lett. 2020, 61, 152254.
doi: 10.1016/j.tetlet.2020.152254 |
|
| [10] |
Chen, Z. W.; Liu, B. T.; Liang, P.; Luo, H. Q.; Zheng, J.; Wen, X. W.; Liu, T. G.; Luo, G. T.; Ye, M. ACS Omega 2019, 4, 281.
doi: 10.1021/acsomega.8b03353 |
| [11] |
Kumar, R. A.; Maheswari, C. U.; Ghantasala, S.; Jyothi, C.; Reddy, K. R. Adv. Synth. Catal. 2011, 353, 401.
doi: 10.1002/adsc.201000580 |
| [1] | Wenting Wei, Zhuangzhuang Li, Wandi Li, Jiaqi Li, Xianying Shi. Green Method for Constructing Phthalides via Oxidative Coupling of Aromatic Acids and Acrylates in Neat Water and Air [J]. Chinese Journal of Organic Chemistry, 2023, 43(3): 1177-1186. |
| [2] | Binghao Huo, Conghui Guo, Zhanhui Xu. Mn(acac)3 Promoted Radical Oxidative Coupling Reaction of Enol Esters with Phosphites to Synthesize β-Ketophosphonates [J]. Chinese Journal of Organic Chemistry, 2023, 43(11): 3989-3996. |
| [3] | Jianming Zhao, Jiashun Zhu, Jiabin Shen, Yilan Zhang, Wanmei Li. Photocatalyzed Oxidative Cross-Coupling Reaction to Access Symmetrical/Unsymmetrical Thiosulfonates [J]. Chinese Journal of Organic Chemistry, 2022, 42(9): 2940-2946. |
| [4] | Wei Chen, Qiang Liu. Recent Advances in the Oxidative Coupling Reaction of Enol Derivatives [J]. Chinese Journal of Organic Chemistry, 2021, 41(9): 3414-3430. |
| [5] | Jin Cai, Xuhua Nong, Bin Wang, Weinü Zeng, Guolei Huang, Ziyi Zhang, Caijuan Zheng. Three New Polyketides from the Culture of Mangrove-Derived FungusEupenicilliumsp. HJ002 [J]. Chinese Journal of Organic Chemistry, 2021, 41(7): 2905-2909. |
| [6] | Yang Zhenhua, Zhu Jianan, Wen Caiyue, Ge Yingxiang, Zhao Shengyin. Recent Advances in Functionalization of Double Bond Based on Maleimides [J]. Chin. J. Org. Chem., 2019, 39(9): 2412-2427. |
| [7] | Chen Wei, Guo Renyu, Gong Jianxian, Yang Zhen. Diastereoselective Construction of All-Carbon Quaternary Stereocenters via Intramolecular Oxidative Cross-Coupling Reaction [J]. Chin. J. Org. Chem., 2019, 39(1): 238-248. |
| [8] | Liu Hong, Dong Huiru, An Rui, Tang Yu, Xu Kaitian, Zhang Yuanming. Synthesis of 1,3-Diaryl or Dialkyl-2H-isoindole-4,7-dione via Barbier-Grignard-Type Reaction [J]. Chin. J. Org. Chem., 2018, 38(8): 2008-2016. |
| [9] | Li Changsheng, Zou Yulong, Jia Changqing, Qin Zhaohai, Ma Yongqiang. Synthesis and Fungicidal Activity of Bridging Ligand Compounds of Azo with 1, 3, 5-Substitued Triazole [J]. Chin. J. Org. Chem., 2018, 38(6): 1500-1506. |
| [10] | Xu Lige, Huang Yi, Liu Binggen, Niu Yunhong, Huo Xing. Progress in Oxidative Coupling Reaction of Olefins, Alkynes and Aromatic Hydrocarbons Catalyzed by Palladium [J]. Chin. J. Org. Chem., 2018, 38(4): 812-824. |
| [11] | Yan Yizhe, Cui Chang, Li Zheng. Recent Advances in Oxidative Coupling Reaction Catalyzed by Low-Valence Iodine [J]. Chin. J. Org. Chem., 2018, 38(10): 2501-2518. |
| [12] | Wang Gang, Guo Yan, Lü Ying, Wang Xicun, Quan Zhengjun. Synthesis of 1,2-Di(pyrimidin-2-yl)disulfides from the Oxidative Coupling and Aromatization of 3,4-Dihydropyrimidin-2-thiones by Using 2,3-Dichloro-5,6-dicyano-1,4-benzoquinone as Oxidant [J]. Chin. J. Org. Chem., 2016, 36(6): 1375-1381. |
| [13] | Zhang Xiang, Cong Ying, Lin Guangyu, Guo Xuliang, Cao Yang, Lei Kunhua, Du Yunfei. Recent Advances of the Application of Organoiodine(Ⅲ) Reagents in the Construction of Heterocyclic Compounds [J]. Chin. J. Org. Chem., 2016, 36(11): 2513-2529. |
| [14] | Zhang Jian, Lu Qingquan, Liu Chao, Lei Aiwen. Recent Advances in Oxidative Coupling Reactions [J]. Chin. J. Org. Chem., 2015, 35(4): 743-759. |
| [15] | Zhang Ke, Xu Xiuhua, Qing Fengling. Recent Advances of Direct Trifluoromethylthiolation [J]. Chin. J. Org. Chem., 2015, 35(3): 556-569. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||