Chinese Journal of Organic Chemistry ›› 2022, Vol. 42 ›› Issue (4): 1111-1122.DOI: 10.6023/cjoc202109052 Previous Articles Next Articles
ARTICLES
高娜a, 初晓辉a, 刘洋b, 李家柱b,*( ), 王进军a,b,*(
), 王进军a,b,*( )
)
收稿日期:2021-09-30
修回日期:2021-12-29
发布日期:2022-01-20
通讯作者:
李家柱, 王进军
基金资助:
Na Gaoa, Xiaohui Chua, Yang Liub, Jiazhu Lib( ), Jinjun Wanga,b(
), Jinjun Wanga,b( )
)
Received:2021-09-30
Revised:2021-12-29
Published:2022-01-20
Contact:
Jiazhu Li, Jinjun Wang
Supported by:Share
Na Gao, Xiaohui Chu, Yang Liu, Jiazhu Li, Jinjun Wang. Regio- and Stereoselective Aryl(aroyl)methylenenation of Pyropheophorbide and Syntheses of Chlorophyllous Chlorin Derivatives[J]. Chinese Journal of Organic Chemistry, 2022, 42(4): 1111-1122.



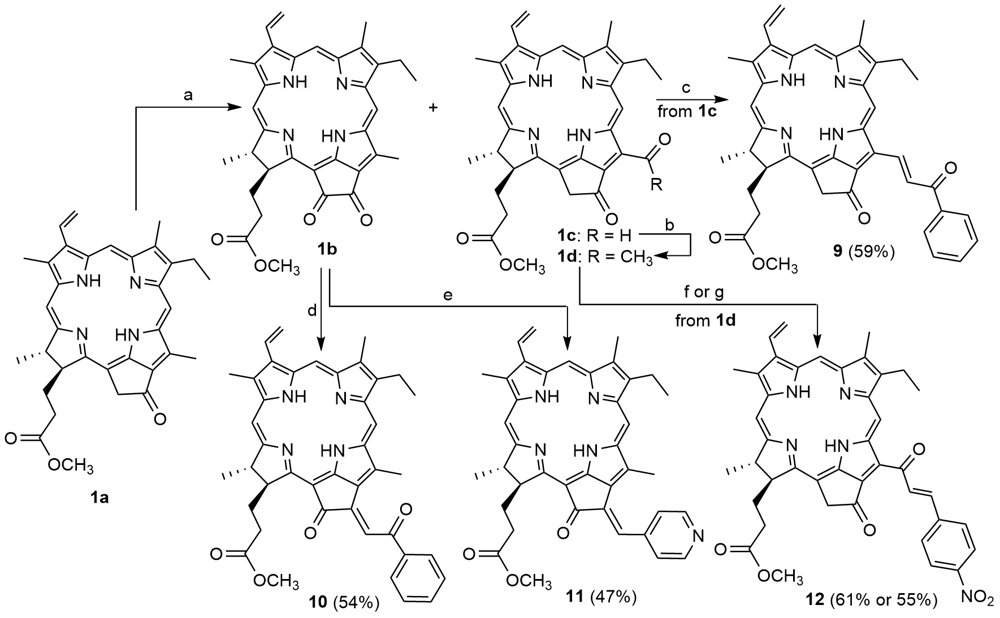
| [1] |
(a) Mori, H.; Tanaka, T.; Osuka, A. J. Mater. Chem. C 2013, 1, 2500.
doi: 10.1039/c3tc00932g pmid: 27258218 |
|
(b) Ono, N.; Yamada, H.; Okujima, T. InHandbook of Porphyrin Science, Vol.2, Eds.: Kadish, K. M.; Smith, K. M.; Guilard, R., World Scientific Publishing Company, Singapore, 2012. p. 1-102.
pmid: 27258218 |
|
|
(c) Stępień, M.; Gońka, E.; Żyła, M.; Sprutta, N. Chem. Rev. 2017, 117, 3479.
doi: 10.1021/acs.chemrev.6b00076 pmid: 27258218 |
|
| [2] |
(a) Wang, J.-J. Chin. J. Org. Chem. 2005, 25, 1353. (in Chinese)
|
|
( 王进军, 有机化学, 2005, 25, 1353.)
|
|
|
(b) Ethirajan, M.; Joshi, P.; William, W. H.; Ohkubo, K.; Fukuzumi, S.; Pandey, R. K. Org. Lett. 2011, 8, 1956.
|
|
|
(c) Bellnier, D. A.; Greco, W. R.; Loewen, G. M.; Nava, H.; Oseroff, A. R.; Pandey, R. K.; Tsuchida, T.; Dougherty, T. J. Cancer. Res. 2003, 63, 1806;
|
|
| [3] |
(a) Ethirajan, M.; Chen, P.; Ohulchanskyy, T. Y.; Goswami, L. N.; Gupta, A.; Srivatsan, A.; Dobhal, M. P.; Missert, J. R.; Prasad, P. N.; Kadish, K. M. Chem.-Eur. J. 2013, 19, 6670.
doi: 10.1002/chem.201203867 |
|
(b) Pandey, R. K.; Goswami, L. N.; Chen, Y.; Gryshuk, A.; Missert, J. R.; Oseroff, A.; Dougherty, T. J. Lasers Surg. Med. 2006, 467, 445.
|
|
|
(c) Tamiaki, H.; Wada, A.; Matsubara, S. J. Photochem. Photobiol., A 2018, 353, 581.
|
|
| [4] |
(a) Pandey, S. K.; Zheng, X.; Morgan, J.; Missert, J. R.; Liu, T.-H.; Shibata, M.; Bellnier, D. A.; Oseroff, A. R.; Henderson, B. W.; Dougherty, T. J.; Pandey, R. K. Mol. Pharmaceutics 2007, 4, 448.
doi: 10.1021/mp060135x |
|
(b) Kozyrey, A. N.; Chen, Y.-H.; Goswami, L. N.; Tabaczynaki, W. A.; Pandey, R. K. J. Org. Chem. 2006, 71, 1949.
doi: 10.1021/jo052334i |
|
|
(c) Duan, S.; Dall’Agnese, C.; Chen, G.; Wang, X.-F.; Tamiaki, H.; Yamamoto, Y.; Ikeuchi, T.; Sasaki, S. ACS Energy Lett. 2018, 3, 1708.
doi: 10.1021/acsenergylett.8b00797 |
|
| [5] |
(a)Ding Y.; Zhu, W.-H.; Xie, Y. Chem. Rev. 2017, 117, 2203.
doi: 10.1021/acs.chemrev.6b00021 |
|
(b) Li, M.; Wei, P.; Ishida, M.; Li, X.; Savage, M.; Guo, R. Angew. Chem., Int. Ed. 2016, 55, 3063.
doi: 10.1002/anie.201510879 |
|
|
(c) Ding, Y.; Tang, Y.; Zhu, W.; Xie, Y. Chem. Soc. Rev. 2015, 44, 1101.
doi: 10.1039/C4CS00436A |
|
|
(d) Xie, Y.; Tang, Y.; Wu, W.; Wang, Y.; Liu, J.; Li, X. J. Am. Chem. Soc. 2015, 137, 14055.
doi: 10.1021/jacs.5b09665 |
|
| [6] |
Senge, M. J. Photochem. Photobiol. B 1992, 16, 3.
doi: 10.1016/1011-1344(92)85150-S |
| [7] |
(a) Zhang, S.-G.; Li, J.-Z.; Zhang, P.; Qi, C.-X.; Wang, J.-J. Chin. J. Org. Chem. 2015, 35, 1060. (in Chinese)
doi: 10.6023/cjoc201408009 |
|
( 张善国, 李家柱, 张朋, 祁彩霞, 王进军, 有机化学, 2015, 35, 1060.)
|
|
|
(b) Zhang, Z.; Xu, X.-S.; Li, Y.-L.; Li, J.-Z.; Wang, J.-J. Chin. J. Org. Chem. 2018, 38, 2993. (in Chinese)
doi: 10.6023/cjoc201804048 |
|
|
( 张珠, 徐希森, 李彦龙, 李家柱, 王进军, 有机化学, 2018, 38, 2993.)
|
|
|
(c) Wang, J.-J.; Li, J.-Z.; Li, Y.-W.; Jakus, J.; Shim, Y. K. J. Porphyrins Phthalocyanines 2010, 14, 860.
|
|
| [8] |
(a) Takahashi, T.; Ogasawara, S.; Shinozaki, Y.; Tamiaki, H. Bull. Chem. Soc. Jpn. 2020, 93, 467.
doi: 10.1246/bcsj.20190367 |
|
(b) Kozyrev, A. N.; Suresh, V.; Das, S.; Senge, M. O.; Shibata, M.; Doughertya, T. J.; Pandeya, R. K. Tetrahedron 2000, 56, 3353.
doi: 10.1016/S0040-4020(00)00256-8 |
|
|
(c) Chen, Y.; Sajjad, M.; Wang, Y..; Batt, C.; Nabi, H. A.; Pandey, R. K. ACS Med. Chem. Lett. 2011, 2, 136.
doi: 10.1021/ml100211g |
|
| [9] |
(a) Zhang, Z.; Li, J.-Z.; Wang, X.-Y.; Ma, J.-H.; Wang, X.; Wang, J.-J. Chin. J. Org. Chem. 2020, 40, 2895. (in Chinese)
doi: 10.6023/cjoc202004054 |
|
( 张珠, 李家柱, 王欣悦, 马计划, 王旭, 王进军, 有机化学, 2020, 40, 2895.)
|
|
|
(b) Zhang, Z.; Zhao, Y.; Wang, X.-Y.; Li, J.-Z.; Wang, J.-J. Chin. J. Org. Chem. 2021, 41, 1177. (in Chinese)
doi: 10.6023/cjoc202006080 |
|
|
( 张珠, 赵雨, 王欣悦, 李家柱, 王进军, 有机化学, 2021, 41, 1177.)
|
|
|
(c) Jiang, Q.-Y.; Zhang, Z.; Liu, Y.; Yao, N.-N.; Wang, J-J. Chin. J. Org. Chem. 2017, 37, 1814. (in Chinese)
doi: 10.6023/cjoc201610045 |
|
|
( 姜齐永, 张珠, 刘洋, 姚楠楠, 王进军, 有机化学, 2017, 37, 1814.)
|
|
| [10] |
(a) Wang, L.-M.; Wang, Z.; Yang, Z.; Jin, Y.-X.; Wang, J.-J. Chin. J. Org. Chem. 2012, 32, 2154. (in Chinese)
doi: 10.6023/cjoc1201202 |
|
( 王鲁敏, 王振, 杨泽, 金英学, 王进军, 有机化学, 2012, 32, 2154.)
|
|
|
(b) Liu, Y.; Xu, X.-S.; Li, J.-Z.; Yin, J.-G.; Qi, C.-X.; Wang, J.-J. Chin. J. Org. Chem. 2014, 34, 552. (in Chinese)
doi: 10.6023/cjoc201310002 |
|
|
( 刘洋, 徐希森, 李家柱, 殷军港, 祁彩霞, 王进军, 有机化学, 2014, 34, 552.)
|
|
|
(c) Gao, N.; Li, J.-Z.; Li, Y.-L.; Wang, Z.; Wang, J.-J. Chin. Chem. Lett. 2016, 27, 789.
doi: 10.1016/j.cclet.2016.01.008 |
|
|
(d) Wu, H.-Q.; Wang, X.-M.; Liu, Y.; Zhao, Y.; Shim, Y.-K.; Yoon, I.; Xu, X.-M.; Li, J.-Z. Bull. Korean Chem. Soc. 2020, 41, 504.
doi: 10.1002/bkcs.11998 |
|
| [11] |
(a) Wang, L.-M.; Wang, P.; Liu, C.; Jin, Y.-X.; Wang, J.-J. Chin. J. Org. Chem. 2012, 32, 1700. (in Chinese)
doi: 10.6023/cjoc1202055 |
|
( 王鲁敏, 王朋, 刘超, 金英学, 王进军, 有机化学, 2012, 32, 1700.)
|
|
|
(b) Ji, J.-Y.; Yin, J.-G.; Zhang, Q.; Liu, C.; Qi, C.-X.; Wang, J.-J. Chin. J. Org. Chem. 2014, 34, 2047. (in Chinese)
doi: 10.6023/cjoc201402041 |
|
|
( 纪建业, 殷军港, 张千, 刘超, 祁彩霞, 王进军, 有机化学, 2014, 34, 2047.)
|
|
|
(c) Shoji, S.; Nomura, Y.; Tamiaki, H. Tetrahedron 2020, 76, 131130.
doi: 10.1016/j.tet.2020.131130 |
|
| [12] |
(a) Li, J.-Z.; Zhang, P.; Yao, N.-N.; Zhao, L.-L.; Wang, J.-J.; Shim, Y.-K. Tetrahedron Lett. 2014, 55, 1086.
doi: 10.1016/j.tetlet.2013.12.093 |
|
(b) Wang, J.-J.; Li, J.-Z.; Jakus., J.; Shim, Y. K. J. Porphyrins Phthalocyanines 2012, 16, 122.
doi: 10.1142/S1088424611004403 |
|
|
(c) Takahashi, T.; Ogasawara, S.; Shinozaki, Y.; Tamiaki, H. Bull. Chem. Soc. Jpn. 2020, 93, 467.
doi: 10.1246/bcsj.20190367 |
|
| [13] |
(a) Li, J.; He, N.; Liu, Y.; Zhang, Z.; Zhang, X.; Han, X.; Gai, Y.; Liu, Y.; Yin, J.; Wang, J. Dyes Pigm. 2017, 146, 189.
doi: 10.1016/j.dyepig.2017.07.005 |
|
(b) Nyman, E. S.; Hynninen, P. H. J. Photochem. Photobiol. B 2004, 73, 1.
doi: 10.1016/j.jphotobiol.2003.10.002 |
|
| [14] |
(a) Wang, P. M.S. Thesis, Yantai University, Yantai, 2009. (in Chinese)
|
|
( 王朋, 硕士论文, 烟台大学, 烟台, 2009.)
|
|
|
(b) Yang, X.-Y.; Yin, J.-G.; Jin, Y.-X.; Qi, C.-X.; Wang, J.-J. Chin. J. Org. Chem. 2014, 34, 2279. (in Chinese)
doi: 10.6023/cjoc201403067 |
|
|
( 杨晓英, 殷军港, 金英学, 祁彩霞, 王进军, 有机化学, 2014, 34, 2279.)
|
|
|
(c) Zhang, Z.; jiang, Q.-Y.; Zhang, Q.; Wu, J.; Wang, J.-J. Chin. J. Org. Chem. 2015, 35, 1929. (in Chinese)
doi: 10.6023/cjoc201503050 |
|
|
( 张珠, 姜齐永, 张千, 武进, 王进军, 有机化学, 2015, 35, 1929.)
|
|
|
(d) Li, Y. -L.; Li, J. Z.; Zhang, S.-G, Wang, J.-J. Simpson, D. J. J. Chin. J. Org. Chem. 2016, 36, 562. (in Chinese)
doi: 10.6023/cjoc201508032 |
|
|
( 李彦龙, 李家柱, 张善国, 王进军, 有机化学, 2016, 36, 562.)
|
|
| [15] |
Smith, K. M.; Gogg, D. A.; Simpson, D. J. J. Am. Chem. Soc. 1985, 107, 4946.
doi: 10.1021/ja00303a021 |
| [16] |
Wang, J.-J.; Zhang, P.; Wang, P.; Chen, G.-L.; Li, F.-G. Chin. J. Org. Chem. 2010, 30, 1192. (in Chinese)
doi: 10.1002/cjoc.201290014 |
|
( 王进军, 张朋, 王朋, 陈冠龙, 有机化学, 2010, 30, 1192.)
|
|
| [17] |
Wang, J.-J.; Wang, P.; Li, J.-Z.; Jakus, J.; Shin, Y.-K. Bull. Korean Chem. Soc. 2011, 32, 3473.
doi: 10.5012/bkcs.2011.32.9.3473 |
| [18] |
Liu, C. M.S. Thesis, Yantai University, Yantai, 2010. (in Chinese)
|
|
( 刘超, 硕士论文, 烟台大学, 烟台, 2010.)
|
|
| [19] |
Mysliwiec, D.; Donnio, B.; Chmielewski, P. J.; Heinrich, B.; Stepien, M. J. Am. Chem. Soc. 2012, 134, 4822.
doi: 10.1021/ja210991f |
| [20] |
Tokuji. S.; Takahashi. Y.; Shinmori. H.; Shinokubo. H.; Osuka. A. Chem. Commun. 2009, 1028.
|
| [21] |
(a) Akita, M.; Hiroto, S.; Shinokubo, H. Angew. Chem., Int. Ed. 2012, 51, 2894.
doi: 10.1002/anie.201108037 |
|
(b) Chen, Y.-H.; Li, L.-G.; Pankey, R.-K. Curr. Org. Chem. 2004, 8, 1107
|
|
| [22] |
Li, J. Z.; Liu, Y.; Xu, X.-S.; Li, Y.-L.; Zhang, S.-G.; Toon, I.; Shim, Y. K.; Wang, J.-J.; Yin, J.-G. Org. Biomol. Chem. 2015, 13, 1992.
doi: 10.1039/C4OB02491E |
| [1] | Jiantao Zhang, Cong Zhang, Nuolin Mo, Jiating Luo, Lianfen Chen, Weibing Liu. Research Progress in Radical Addition Reaction of Alkenes Involving Chloroform [J]. Chinese Journal of Organic Chemistry, 2023, 43(9): 3098-3106. |
| [2] | Fen Huang, Weiwei Luo, Jun Zhou. Research Progress of Polychloroalkylation Based on C—H Bond Cleavage [J]. Chinese Journal of Organic Chemistry, 2023, 43(7): 2368-2390. |
| [3] | Yun Zhao, Yanfang Li, Rongxiao Li, Yaqing Wang, Xiaoxia Fan. A New Fluorescent Probe for Hypochlorous Acid Based on Chlorinium Ions Recognition Mechanism and Its Bioimaging Research in Living Cells [J]. Chinese Journal of Organic Chemistry, 2021, 41(5): 1974-1981. |
| [4] | Zhu Zhang, Yu Zhao, Xinyue Wang, Jiazhu Li, Jinjun Wang. Syntheses of Chlorophyllous Chlorin Derivatives with Aromatic Ring-Fused Imidazole Structural Unit [J]. Chinese Journal of Organic Chemistry, 2021, 41(3): 1177-1186. |
| [5] | Yaoxin Wang, Chen Cui, Xiaohui Yang. Recent Advances in Hydrochlorination of Alkenes [J]. Chinese Journal of Organic Chemistry, 2021, 41(10): 3808-3815. |
| [6] | Zhang Zhu, Li Jiazhu, Wang Xinyue, Ma Jihua, Wang Xu, Wang Jinjun. Nitration (Nitroalkylation) of Pheophorbide and Synthesis of Chlorophyllous Chlorin Derivatives [J]. Chinese Journal of Organic Chemistry, 2020, 40(9): 2895-2903. |
| [7] | Zhu Ye, Huang Jinwen, Yang Xianjin. Chlorination of Anilide by Pd(OAc)2/N-Chloro-N-fluorobenzene- sulfonylamide [J]. Chin. J. Org. Chem., 2019, 39(6): 1665-1671. |
| [8] | Zong Wuzhong, Hu Yulai, Wang Xiaoping, Liu Jiaxin, Huang Danfeng, Wang Kehu. Study on the Chlorination Reaction of Hydrazones by Using of Trichloroisocyanuric Acid as Chloride Source [J]. Chin. J. Org. Chem., 2019, 39(5): 1396-1403. |
| [9] | Su Yingpeng, Shi Ya, Chang Bingbing, Wu Lili, Chong Siying, Zhang Weigang, Huang Danfeng, Wang Kehu, Hu Yulai. Chlorination of α-Cyanoketones and 1, 3-Dicarbonyl Compounds by Using Trichloroisocyanuric Acid as Chlorine Source [J]. Chin. J. Org. Chem., 2018, 38(6): 1454-1461. |
| [10] | Zhang Zhu, Li Jiazhu, Zhang Shanguo, Wang Zhen, Wang Jinjun. Heterocyclization for the Structures on the Periphery of Py-ropheophorbide and Synthesis of Chlorophyll Derivatives [J]. Chin. J. Org. Chem., 2018, 38(12): 3250-3259. |
| [11] | Liu Lifen, Zhang Xiao, Xie Xin, Li Ruihan, Gao Congjie. Modification of Polyimide-Urethane Reverse Osimosis Composite Membrane Based on NaA Zeolite [J]. Chin. J. Org. Chem., 2018, 38(11): 3127-3136. |
| [12] | Zhang Zhu, Xu Xisen, Li Yanlong, Li Jiazhu, Wang Jinjun. Rearrangement Reactions of Pyropheophorbide with Diazoalkane and Synthesis of Chlorophyll Derivatives [J]. Chin. J. Org. Chem., 2018, 38(11): 2993-3001. |
| [13] | Jiang Qiyong, Zhang Zhu, Liu Yang, Yao Nannan, Wang Jinjun. Synthesis of Chlorin Aldehydes with Chlorophyllous Skeleton and Their Interactions with Protein [J]. Chin. J. Org. Chem., 2017, 37(7): 1814-1823. |
| [14] | Ren Shaobo, Zhang Haifeng, Zhang Jian, Zhang Wei, Liu Yunkui. Dichlorinating Reaction of N-Chlorosuccinimide with Isonitriles for the Synthesis of N-Phenyldichloroimides [J]. Chin. J. Org. Chem., 2016, 36(8): 1954-1957. |
| [15] | Chen Xiaowei, Chong Siying, Du Ganggang, Huang Danfeng, Wang Kehu, Su Yingpeng, Hu Yulai. Diacetoxyiodobenzene Promoted Chlorination of Silyl Enol Ether of Aryl Ketones [J]. Chin. J. Org. Chem., 2016, 36(5): 1028-1033. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||