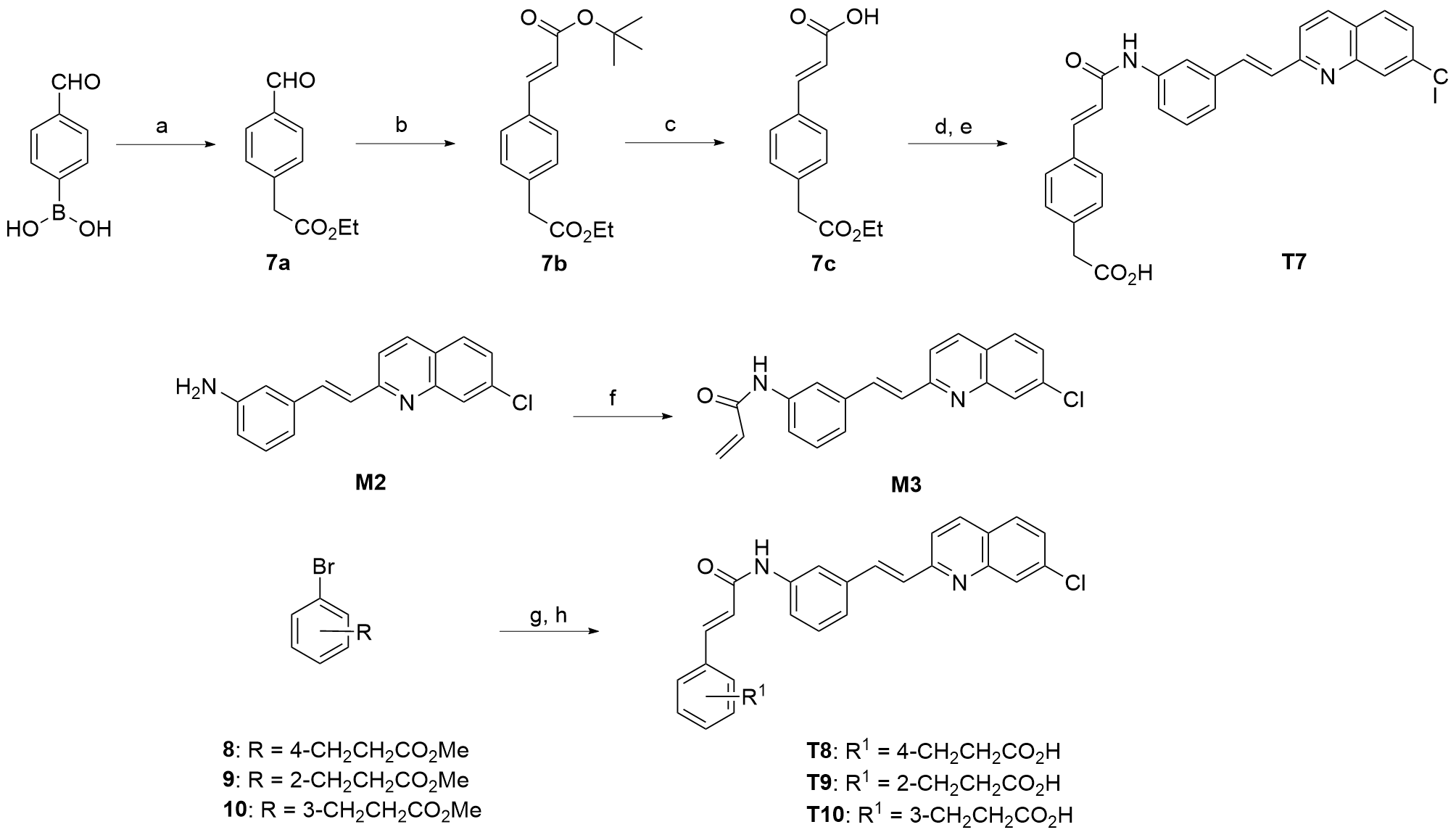
Chinese Journal of Organic Chemistry ›› 2024, Vol. 44 ›› Issue (1): 259-276.DOI: 10.6023/cjoc202306001 Previous Articles Next Articles
ARTICLES
王婧怡a,b, 刘金羽c, 陈东升b, 陈华燕b, 谢欣*( ), 南发俊a,b,c,*(
), 南发俊a,b,c,*( )
)
收稿日期:2023-06-01
修回日期:2023-07-18
发布日期:2023-09-08
基金资助:
Jingyi Wanga,b, Jinyu Liuc, Dongsheng Chenb, Huayan Chenb, Xin Xie( ), Fajun Nana,b,c(
), Fajun Nana,b,c( )
)
Received:2023-06-01
Revised:2023-07-18
Published:2023-09-08
Contact:
*E-mail: Supported by:Share
Jingyi Wang, Jinyu Liu, Dongsheng Chen, Huayan Chen, Xin Xie, Fajun Nan. Design, Synthesis, and Structure-Activity Relationship of Novel Potent and Highly Selective Cysteinyl Leukotriene Receptor 1 (CysLT1R) Antagonists[J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 259-276.
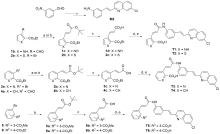

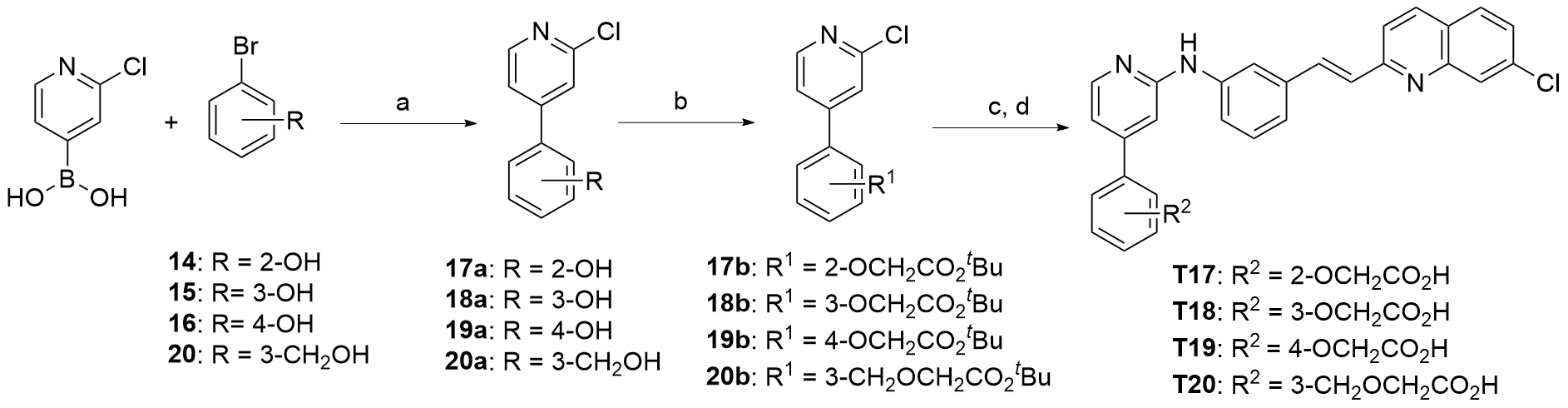
| Compd. | R | IC50a/(μmol•L-1) | Compd. | R | IC50a/(μmol•L-1) |
|---|---|---|---|---|---|
| 17g | | 0.13±0.023 | T4 | | 0.94±0.098 |
| T1 | | 0.35±0.12 | T5 | | 0.20±0.10 |
| T2 | | 0.85±0.14 | T6 | | 0.10±0.014 |
| T3 | | 2.77±0.50 | Montelukast | 0.057±0.029 |
| Compd. | R | IC50a/(μmol•L-1) | Compd. | R | IC50a/(μmol•L-1) |
|---|---|---|---|---|---|
| 17g | | 0.13±0.023 | T4 | | 0.94±0.098 |
| T1 | | 0.35±0.12 | T5 | | 0.20±0.10 |
| T2 | | 0.85±0.14 | T6 | | 0.10±0.014 |
| T3 | | 2.77±0.50 | Montelukast | 0.057±0.029 |

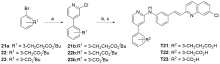
| Compd. | R | IC50a/(μmol•L-1) | Compd. | R | IC50a/(μmol•L-1) |
|---|---|---|---|---|---|
| T7 | | 0.24±0.098 | T9 | | 0.0066±0.0023 |
| T8 | | 0.038±0.0060 | T10 | | 0.037±0.0090 |
| Compd. | R | IC50a/(μmol•L-1) | Compd. | R | IC50a/(μmol•L-1) |
|---|---|---|---|---|---|
| T7 | | 0.24±0.098 | T9 | | 0.0066±0.0023 |
| T8 | | 0.038±0.0060 | T10 | | 0.037±0.0090 |
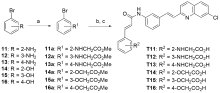
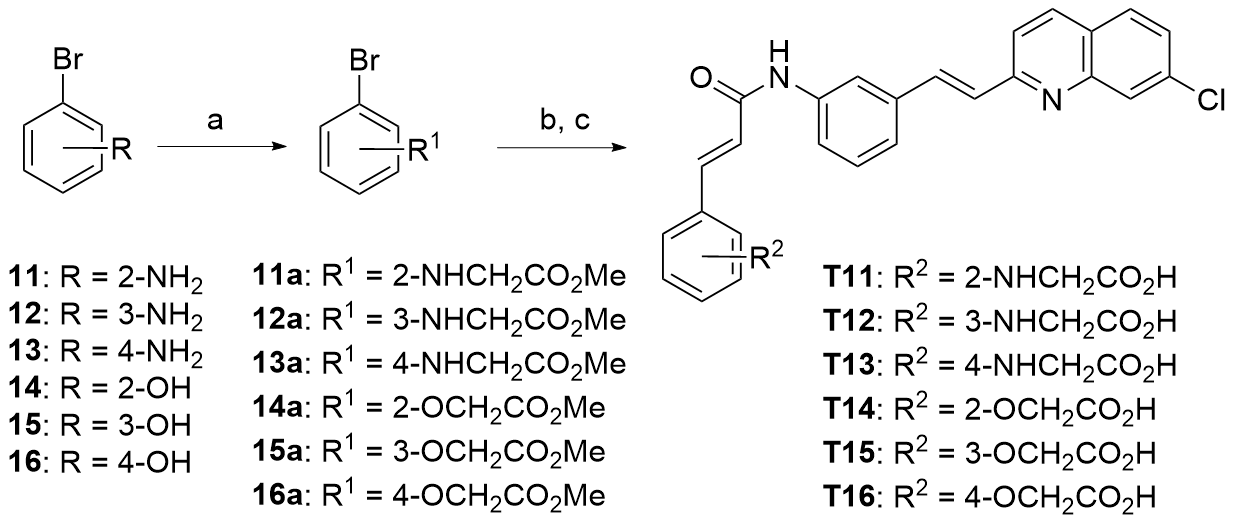
| Compd. | R | IC50a/(μmol•L-1) | Compd. | R | IC50a/(μmol•L-1) |
|---|---|---|---|---|---|
| T11 | | 0.068±0.013 | T14 | | 0.059±0.022 |
| T12 | | 0.13±0.032 | T15 | | 0.045±0.012 |
| T13 | | 0.075±0.00078 | T16 | | 0.039±0.0097 |
| Compd. | R | IC50a/(μmol•L-1) | Compd. | R | IC50a/(μmol•L-1) |
|---|---|---|---|---|---|
| T11 | | 0.068±0.013 | T14 | | 0.059±0.022 |
| T12 | | 0.13±0.032 | T15 | | 0.045±0.012 |
| T13 | | 0.075±0.00078 | T16 | | 0.039±0.0097 |


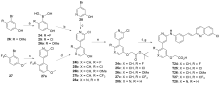
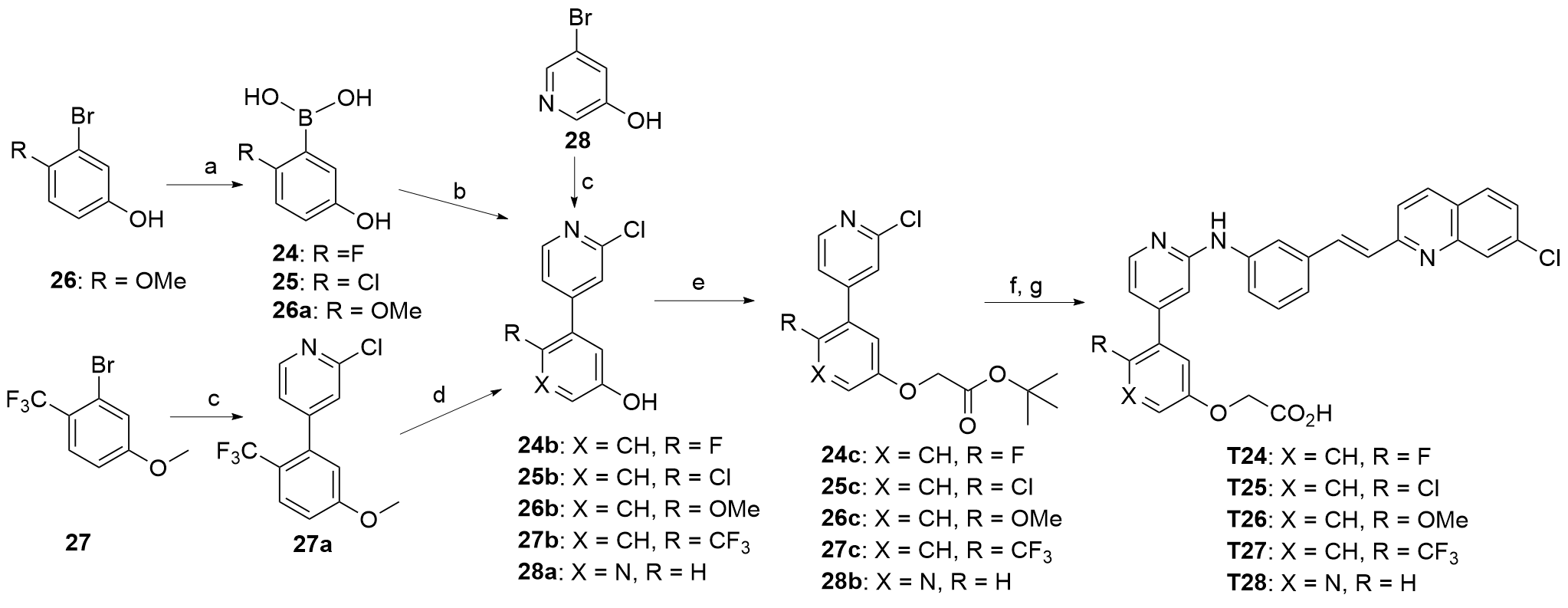

| Compd. | R | IC50a/(μmol•L-1) | Compd. | R | IC50a/(μmol•L-1) |
|---|---|---|---|---|---|
| T17 | | 0.27±0.038 | T24 | | 0.075±0.0055 |
| T18 | | 0.070±0.0082 | T25 | | 0.60±0.024 |
| T19 | | 0.24±0.012 | T26 | | 1.37±0.037 |
| T20 | | 0.59±0.25 | T27 | | 0.0081±0.002 |
| Compd. | R | IC50a/(μmol•L-1) | Compd. | R | IC50a/(μmol•L-1) |
| T21 | | 0.13±0.041 | T28 | | 0.0025±0.0004 |
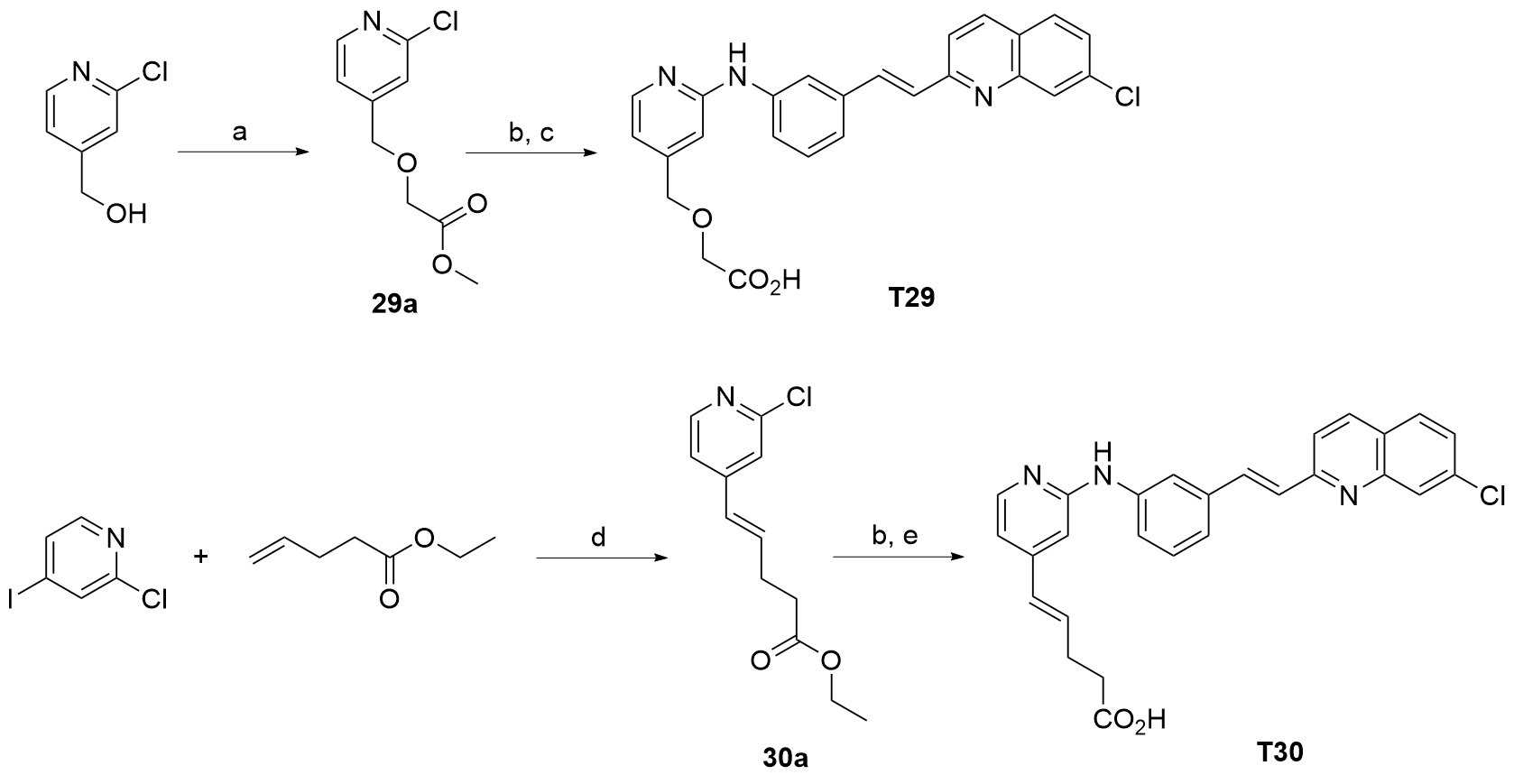
| T22 | | 0.39±0.070 | T29 | | 0.0057±0.001 |
| T23 | | 0.10±0.026 | T30 | | 0.010±0.0024 |
| Compd. | R | IC50a/(μmol•L-1) | Compd. | R | IC50a/(μmol•L-1) |
|---|---|---|---|---|---|
| T17 | | 0.27±0.038 | T24 | | 0.075±0.0055 |
| T18 | | 0.070±0.0082 | T25 | | 0.60±0.024 |
| T19 | | 0.24±0.012 | T26 | | 1.37±0.037 |
| T20 | | 0.59±0.25 | T27 | | 0.0081±0.002 |
| Compd. | R | IC50a/(μmol•L-1) | Compd. | R | IC50a/(μmol•L-1) |
| T21 | | 0.13±0.041 | T28 | | 0.0025±0.0004 |
| T22 | | 0.39±0.070 | T29 | | 0.0057±0.001 |
| T23 | | 0.10±0.026 | T30 | | 0.010±0.0024 |
| Compd. | IC50a/(μmol•L-1) | |
|---|---|---|
| CysLT1R | CysLT2R | |
| 17g | 0.13±0.023 | >100 |
| T9 | 0.0066±0.0023 | >100 |
| T27 | 0.0081±0.002 | 8.75±1.14 |
| T28 | 0.0025±0.0004 | >100 |
| T29 | 0.0057±0.001 | >100 |
| T30 | 0.010±0.0024 | >100 |
| Montelukast | 0.057±0.029 | >100 |
| Compd. | IC50a/(μmol•L-1) | |
|---|---|---|
| CysLT1R | CysLT2R | |
| 17g | 0.13±0.023 | >100 |
| T9 | 0.0066±0.0023 | >100 |
| T27 | 0.0081±0.002 | 8.75±1.14 |
| T28 | 0.0025±0.0004 | >100 |
| T29 | 0.0057±0.001 | >100 |
| T30 | 0.010±0.0024 | >100 |
| Montelukast | 0.057±0.029 | >100 |
| [1] |
Samuelsson, B.; Borgeat, P.; Hammarstroem, S.; Murphy, R. C. Prostaglandins 1979, 17, 785.
doi: 10.1016/0090-6980(79)90052-2 |
| [2] |
Peters-Golden, M.; Brock, T. G. Prostaglandins, Leukotrienes Essent. Fatty Acids 2003, 69, 99.
doi: 10.1016/S0952-3278(03)00070-X |
| [3] |
Samuelsson, B.; Dahlen, S. E.; Lindgren, J. A.; Rouzer, C. A.; Serhan, C. N. Science 1987, 237, 1171.
doi: 10.1126/science.2820055 |
| [4] |
Singh, R. K.; Gupta, S.; Dastidar, S.; Ray, A. Pharmacology 2010, 85, 336.
doi: 10.1159/000312669 |
| [5] |
Maekawa, A.; Kanaoka, Y.; Xing, W.; Austen, K. F. Proc. Natl. Acad. Sci. U. S. A. 2008, 105, 16695.
doi: 10.1073/pnas.0808993105 |
| [6] |
Kanaoka, Y.; Maekawa, A.; Austen, K. F. J. Biol Chem. 2013, 288, 10967.
doi: 10.1074/jbc.C113.453704 |
| [7] |
Lynch, K. R.; O'Nneill, G. P.; Liu, Q.-Y.; Im, D. S.; Sawyer, N.; Metters, K. M.; Coulombe, N.; Abramovitz, M.; Figueroa, D. J.; Zeng, Z.-Z.; Connolly, B. M.; Bai, C.; Austin, C. P.; Chateauneuf, A.; Stocco, R.; Greig, G. M.; Kargman, S.; Hooks, S. B.; Hosfield, E.; Williams, D. L. Jr.; Ford-Hutchinson, A. W.; Thomas Caskey, C.; Evans, J. F. Nature 1999, 399, 789.
doi: 10.1038/21658 |
| [8] |
Capra, V.; Thompson, M. D.; Sala, A.; Cole, D. E.; Folco, G.; Rovati, G. E. Med. Res. Rev. 2007, 27, 469.
doi: 10.1002/med.v27:4 |
| [9] |
Heise, C. E.; O'Dowd, B. F.; Figueroa, D. J.; Sawyer, N.; Nguyen, T.; Im, D. S.; Stocco, R.; Bellefeuille, J. N.; Abramovitz, M.; Cheng, R.; Williams, D. L., Jr.; Zeng, Z.-Z.; Liu, Q.-Y.; Ma, L.; Clements, M. K.; Coulombe, N.; Liu, Y.; Austin, C. P.; George, S. R.; O'Neill, G. P.; Metters, K. M.; Lynch, K. R.; Evans, J. F. J. Biol. Chem. 2000, 275, 30531.
doi: 10.1074/jbc.M003490200 |
| [10] |
Singh, R. K.; Tandon, R.; Dastidar, S. G.; Ray, A. J. Asthma 2013, 50, 922.
doi: 10.3109/02770903.2013.823447 |
| [11] |
Montuschi, P. Pharmaceuticals 2010, 3, 1792.
doi: 10.3390/ph3061792 |
| [12] |
Liu, M.; Yokomizo, T. Allergol. Int. 2015, 64, 17.
doi: 10.1016/j.alit.2014.09.001 |
| [13] |
Bellamkonda, K.; Satapathy, S. R.; Douglas, D.; Chandrashekar, N.; Selvanesan, B. C.; Liu, M.-H.; Savari, S.; Jonsson, G.; Sjolander, A. Cancer Lett. 2018, 437, 13.
doi: 10.1016/j.canlet.2018.08.019 |
| [14] |
Savari, S.; Liu, M.-H.; Zhang, Y.; Sime, W.; Sjolander, A. PLoS One 2013, 8, e73466.
|
| [15] |
Tsai, M.-J.; Chang, W.-A.; Tsai, P.-H.; Wu, C. Y.; Ho, Y.-W.; Yen, M.-C.; Lin, Y.-S.; Kuo, P.-L.; Hsu, Y.-L. Int. J. Mol. Sci. 2017, 18, 1353.
doi: 10.3390/ijms18071353 |
| [16] |
Wang, L.-F.; Du, C.-S.; Lv, J.; Wei, W.; Cui, Y.; Xie, X. J. Immunol. 2011, 187, 2336.
doi: 10.4049/jimmunol.1100333 |
| [17] |
Tang, S.-S.; Wang, X.-Y.; Hong, H.; Long, Y.; Li, Y.-Q.; Xiang, G.-Q.; Jiang, L.-Y.; Zhang, H.-T.; Liu, L.-P.; Miao, M.-X.; Hu, M.; Zhang, T.-T.; Hu, W.; Ji, H.; Ye, F.-Y. Neuropharmacology 2013, 65, 182.
doi: 10.1016/j.neuropharm.2012.08.026 |
| [18] |
Tang, S.-S.; Ji, M.-J.; Chen, L.; Hu, M.; Long, Y.; Li, Y.-Q.; Miao, M.-X.; Li, J.-C.; Li, N.; Ji, H.; Chen, X.-J.; Hong, H. Int. J. Neuropsychopharmacol. 2014, 17, 581.
doi: 10.1017/S1461145713001314 |
| [19] |
Dey, M.; Singh, R. K. Pharmacology 2021, 106, 469.
doi: 10.1159/000518359 |
| [20] |
Chen, H.-Y.; Yang, H.; Wang, Z.-L.; Xie, X.; Nan, F.-J. ACS Med. Chem. Lett. 2016, 7, 335.
doi: 10.1021/acsmedchemlett.5b00482 |
| [21] |
Tvaermose-Nielsen, O.; Rachlin, S.; Dannacher, H.; Bjorkling, F.; Kirstein, D.; Bramm, E.; Nielsen, C. K.; Mortensen, J. T.; Binderup, L. Bioorg. Med. Chem. 1997, 5, 415.
|
| [22] |
Liu, J.-Q.; Fang, Z.-X.; Zhang, Q.; Liu, Q.; Bi, X.-H. Angew. Chem., nt. Ed. 2013, 52, 6953.
|
| [23] |
Peng, Z.-Y.; Wang, J.-P.; Cheng, J.; Xie, X.-M.; Zhang, Z.-G. Tetrahedron 2010, 66, 8238.
doi: 10.1016/j.tet.2010.08.047 |
| [24] |
Honda, T.; Terao, T.; Aono, H.; Ban, M. Bioorg. Med. Chem. 2009, 17, 699.
doi: 10.1016/j.bmc.2008.11.060 |
| [25] |
Woo, L. W. L.; Jackson, T.; Putey, A.; Cozier, G.; Leonard, P.; Acharya, K. R.; Chander, S. K.; Purohit, A.; Reed, M. J.; Potter, B. V. L. J. Med. Chem. 2010, 53, 2155.
doi: 10.1021/jm901705h |
| [26] |
Kawate, T.; Iwase, N.; Shimizu, M.; Stanley, S. A.; Wellington, S.; Kazyanskaya, E.; Hung, D. T. Bioorg. Med. Chem. Lett. 2013, 23, 6052.
doi: 10.1016/j.bmcl.2013.09.035 |
| No related articles found! |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||