Chinese Journal of Organic Chemistry ›› 2024, Vol. 44 ›› Issue (3): 951-965.DOI: 10.6023/cjoc202312005 Previous Articles Next Articles
ARTICLES
韩璐瑶a, 胡硕真a, 郭庆春b, 郭红永b, 高照群a, 许颖a, 张新胜a,*( )
)
收稿日期:2023-12-06
修回日期:2024-02-23
发布日期:2024-04-02
Luyao Hana, Shuozhen Hua, Qingchun Guob, Hongyong Guob, Zhaoqun Gaoa, Ying Xua, Xinsheng Zhanga( )
)
Received:2023-12-06
Revised:2024-02-23
Published:2024-04-02
Contact:
*E-mail: xszhang@ecust.edu.cn
Share
Luyao Han, Shuozhen Hu, Qingchun Guo, Hongyong Guo, Zhaoqun Gao, Ying Xu, Xinsheng Zhang. Study on Electrooxidation of Benzophenone Hydrazone to Diphenyldiazomethane[J]. Chinese Journal of Organic Chemistry, 2024, 44(3): 951-965.
| Entry | Electrolyte | pH | |
|---|---|---|---|
| Aqueous solutiona | DMAc/H2Ob | ||
| 1 | (NH4)2CO3 | 10.25 | 10.28 |
| 2 | NH4HCO3 | 8.40 | 8.95 |
| 3 | CH3COONH4 | 7.58 | 8.06 |
| 4 | (NH4)2HPO4 | 8.88 | 4.77 |
| Entry | Electrolyte | pH | |
|---|---|---|---|
| Aqueous solutiona | DMAc/H2Ob | ||
| 1 | (NH4)2CO3 | 10.25 | 10.28 |
| 2 | NH4HCO3 | 8.40 | 8.95 |
| 3 | CH3COONH4 | 7.58 | 8.06 |
| 4 | (NH4)2HPO4 | 8.88 | 4.77 |
| Entry | Electrolyte | Conv.b/% | Yield/% | |||
|---|---|---|---|---|---|---|
| 1c | 2d | 3e | 4f | |||
| 1 | (NH4)2CO3 | 49.4 | 39.5 | 4.8 | 0 | <0.1 |
| 2 | NH4HCO3 | 54.0 | 36.3 | 5.1 | <0.1 | 2.3 |
| 3 | CH3COONH4 | 47.4 | 18.2 | 10.7 | 2.8 | 2.9 |
| 4 | (NH4)2HPO4 | 67.2 | 8.8 | 29.0 | 3.8 | 0.1 |
| Entry | Electrolyte | Conv.b/% | Yield/% | |||
|---|---|---|---|---|---|---|
| 1c | 2d | 3e | 4f | |||
| 1 | (NH4)2CO3 | 49.4 | 39.5 | 4.8 | 0 | <0.1 |
| 2 | NH4HCO3 | 54.0 | 36.3 | 5.1 | <0.1 | 2.3 |
| 3 | CH3COONH4 | 47.4 | 18.2 | 10.7 | 2.8 | 2.9 |
| 4 | (NH4)2HPO4 | 67.2 | 8.8 | 29.0 | 3.8 | 0.1 |
| Entry | Solvent type | Dipole moment/(×10-30 C•m) | Conv.b/% | Yield/% | |||
|---|---|---|---|---|---|---|---|
| 1 c | 2d | 3e | 4f | ||||
| 1 | DMSO | 13.34 | 100.0 | 34.7 | 43.3 | 16.7 | 1.7 |
| 2 | DMF | 12.88 | 100.0 | 66.3 | 16.3 | 6.6 | 1.0 |
| 3 | DMAc | 12.41 | 99.4 | 73.8 | 3.3 | 3.1 | 1.6 |
| 4 | ACN | 11.47 | 73.2 | 41.3 | 3.8 | 0.8 | 10.0 |
| Entry | Solvent type | Dipole moment/(×10-30 C•m) | Conv.b/% | Yield/% | |||
|---|---|---|---|---|---|---|---|
| 1 c | 2d | 3e | 4f | ||||
| 1 | DMSO | 13.34 | 100.0 | 34.7 | 43.3 | 16.7 | 1.7 |
| 2 | DMF | 12.88 | 100.0 | 66.3 | 16.3 | 6.6 | 1.0 |
| 3 | DMAc | 12.41 | 99.4 | 73.8 | 3.3 | 3.1 | 1.6 |
| 4 | ACN | 11.47 | 73.2 | 41.3 | 3.8 | 0.8 | 10.0 |
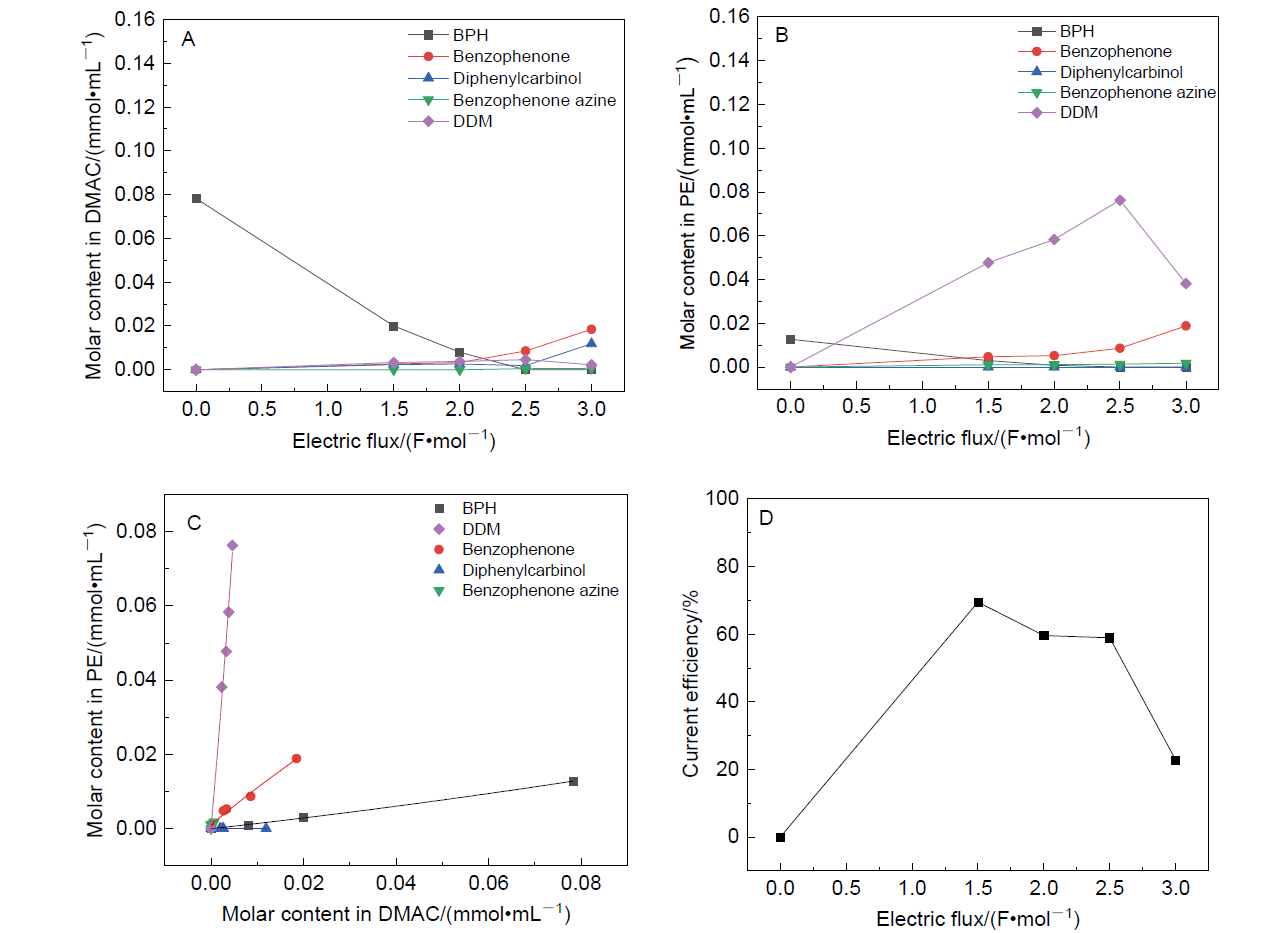
| Entry | Amplification coefficient | Conv.d/% | Current efficiency/% | Yielde/% | |||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||||
| 1a | 1 | 100.0 | 58.9 | 73.7 | 7.6 | 2.9 | 1.2 |
| 2b | 5 | 99.9 | 57.9 | 72.4 | 7.3 | 2.5 | 0.2 |
| 3c | 10 | 100.0 | 56.4 | 70.5 | 7.8 | 2.1 | 0.4 |
| Entry | Amplification coefficient | Conv.d/% | Current efficiency/% | Yielde/% | |||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||||
| 1a | 1 | 100.0 | 58.9 | 73.7 | 7.6 | 2.9 | 1.2 |
| 2b | 5 | 99.9 | 57.9 | 72.4 | 7.3 | 2.5 | 0.2 |
| 3c | 10 | 100.0 | 56.4 | 70.5 | 7.8 | 2.1 | 0.4 |
| Entry | c(BPH)/(mol•L-1) | Conv.b/% | Current efficiency/% | Yield/% | |||
|---|---|---|---|---|---|---|---|
| 1 c | 2d | 3e | 4f | ||||
| 1 | 0.0975 | 100.0 | 58.9 | 73.7 | 7.6 | 2.9 | 1.2 |
| 2 | 0.1951 | 100.0 | 50.8 | 63.4 | 7.4 | 4.1 | 1.4 |
| 3 | 0.4878 | 95.9 | 41.2 | 51.4 | 1.8 | 2.5 | 1.8 |
| 4 | 0.9756 | 91.4 | 39.6 | 49.5 | 0.7 | 1.7 | 2.2 |
| Entry | c(BPH)/(mol•L-1) | Conv.b/% | Current efficiency/% | Yield/% | |||
|---|---|---|---|---|---|---|---|
| 1 c | 2d | 3e | 4f | ||||
| 1 | 0.0975 | 100.0 | 58.9 | 73.7 | 7.6 | 2.9 | 1.2 |
| 2 | 0.1951 | 100.0 | 50.8 | 63.4 | 7.4 | 4.1 | 1.4 |
| 3 | 0.4878 | 95.9 | 41.2 | 51.4 | 1.8 | 2.5 | 1.8 |
| 4 | 0.9756 | 91.4 | 39.6 | 49.5 | 0.7 | 1.7 | 2.2 |
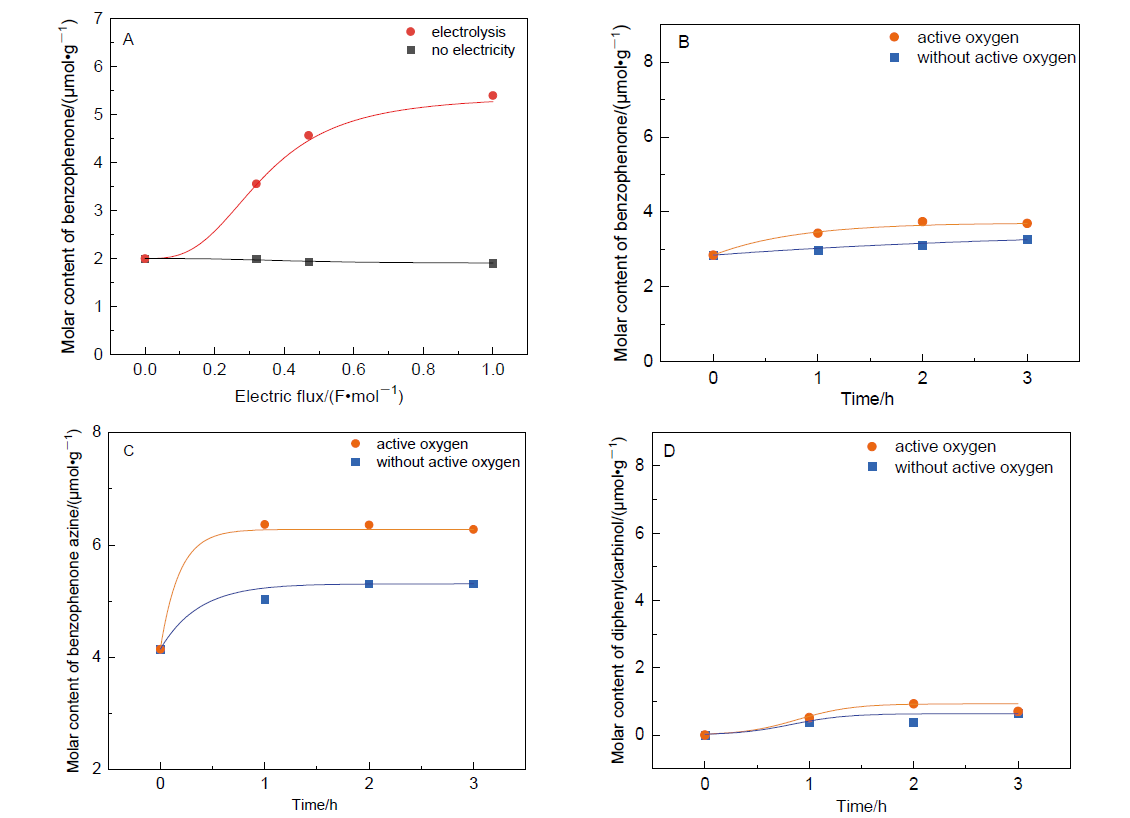
| [1] |
Amy K. F. Chem. Sci. 2015, 6, 752.
doi: 10.1039/C4SC01768D |
| [2] |
Mix K. A.; Aronoff M. R.; Raines R. T. ACS Chem. Biol. 2016, 11, 3233.
doi: 10.1021/acschembio.6b00810 |
| [3] |
Fulton J. R.; Aggarwal V. K.; de Vicente J. Eur. J. Org. Chem. 2005, 2005, 1479.
doi: 10.1002/ejoc.v2005:8 |
| [4] |
David T. E. US 4092306, 1978.
|
| [5] |
Miller J. J. Org. Chem. 1959, 24, 560.
doi: 10.1021/jo01086a603 |
| [6] |
Holton T. L.; Schechter H. J. Org. Chem. 1995, 60, 4725.
doi: 10.1021/jo00120a013 |
| [7] |
Kawahara I.US 5587464, 1996.
|
| [8] |
Perusquía-Hernández C.; Lara-Issasi G. R.; Frontana-Uribe B. A. Tetrahedron Lett. 2013, 54, 3302.
doi: 10.1016/j.tetlet.2013.04.079 |
| [9] |
Guo H.; Niu D. F.; Hu S. Z. J. Electrochem. 2021, 27, 498. (in Chinese)
|
|
( 郭浩, 钮东方, 胡硕真, 电化学, 2021, 27, 498.)
doi: 10.13208/j.electrochem.201203 |
|
| [10] |
Mao L.; Niu D. F.; Hu S. Z. J. Electrochem. 2022, 28, 2107061. (in Chinese)
doi: 10.13208/j.electrochem.210706 |
|
( 毛麟, 钮东方, 胡硕真, 电化学, 2022, 28, 2107061.)
doi: 10.13208/j.electrochem.210706 |
|
| [11] |
Chiba T. J. Org. Chem. 1983, 48, 2968.
doi: 10.1021/jo00166a006 |
| [12] |
Okimoto M.; Numata K.; Tomozawa K. Aust. J. Chem. 2005, 58, 560.
doi: 10.1071/CH05090 |
| [13] |
Tanbouza N. Org. Lett. 2022, 24, 4665.
doi: 10.1021/acs.orglett.2c01803 |
| [14] |
Liu Z.; Sivaguru P.; Zanoni G. Acc. Chem. Res. 2022, 55, 1763.
doi: 10.1021/acs.accounts.2c00186 |
| [15] |
O’Connor, N. R.; Bolgar, P.; Stoltz, B. M. Tetrahedron Lett. 2016, 57, 849.
doi: 10.1016/j.tetlet.2016.01.020 |
| [16] |
Bartlett P. D.; Traylor T. G. J. Am. Chem. Soc. 1962, 84, 3408.
doi: 10.1021/ja00876a041 |
| [17] |
Yu Y.; Sha Q.; Cui H. Org. Lett. 2018, 20, 776.
doi: 10.1021/acs.orglett.7b03912 |
| [18] |
Reader A. M.; Bailey P. S.; White H. M. J. Org. Chem. 1965, 30, 784.
doi: 10.1021/jo01014a032 |
| [19] |
Nojima T.; Ishiguro K.; Sawaki Y. J. Org. Chem. 1997, 62, 6911.
doi: 10.1021/jo9711394 |
| [20] |
Sawaki Y.; Ishiguro K. Tetrahedron Lett. 1984, 25, 1487.
doi: 10.1016/S0040-4039(01)80193-3 |
| [21] |
Adam W.; Zhao C. G.; Saha-Moller C. R.; Jakka K. Oxidation of Organic Compounds by Dioxiranes, John Wiley & Sons., Canada, 2009, pp. 311-662.
|
| [22] |
Chapman O. L.; Hess T. C.; J. Am. Chem. Soc. 1984, 106, 1842.
doi: 10.1021/ja00318a052 |
| [23] |
Ishiguro K.; Hirano Y.; Sawaki Y. J. Org. Chem. 1988, 53, 5397.
doi: 10.1021/jo00258a002 |
| [24] |
Dahn H.; Ballenegger M. Helv. Chim. Acta 1969, 52, 2417.
doi: 10.1002/hlca.v52:8 |
| [25] |
Empel C.; Pei C.; He F. Chem.-Eur. J. 2022, 28, e202104397.
doi: 10.1002/chem.v28.15 |
| [26] |
Nozaki H.; Takaya H.; Moriuti S. Tetrahedron 1968, 24, 3655.
doi: 10.1016/S0040-4020(01)91998-2 |
| [27] |
Abelt C. J.; Pleier J. M. J. Am. Chem. Soc. 1989, 111, 1795.
doi: 10.1021/ja00187a037 |
| [28] |
Quiclet-Sire B.; Zard S. Z. Chem. Commun. 2006, 1831.
|
| [29] |
Barton D. H. R.; O'brien R. E.; Sternhell S. J. Chem. Soc. 1962, 88, 470.
|
| [30] |
Overberger C. G.; Anselme J. P. Org. Chem. 1964, 29, 1188.
doi: 10.1021/jo01028a048 |
| [31] |
Liu K.; Song C.; Lei A. Org. Biomol. Chem. 2018, 16, 2375.
doi: 10.1039/C8OB00063H |
| [32] |
Lian F.; Xu K.; Zeng C. Chem. Rec. 2021, 21, 2290.
doi: 10.1002/tcr.v21.9 |
| [33] |
Tang H. T.; Jia J. S.; Pan Y. M. Org. Biomol. Chem. 2020, 18, 5315.
doi: 10.1039/D0OB01008A |
| [34] |
Dou G. Y.; Jiang Y. Y.; Xu K. Org. Chem. Front. 2019, 6, 2392.
doi: 10.1039/C9QO00552H |
| [35] |
Wang Z. Q.; Meng X. J.; Li Q. Y. Adv. Synth. Catal. 2018, 360, 4043.
doi: 10.1002/adsc.v360.21 |
| [36] |
Mo Z. Y.; Swaroop T. P.; Tong W. Green Chem. 2018, 20, 4428.
doi: 10.1039/C8GC02143K |
| [37] |
Zhang Y. Z.; Mo Z. Y.; Wang H. S. Green Chem. 2019, 21, 3807.
doi: 10.1039/C9GC01201J |
| [38] |
Meng X. J.; Zhong P. F.; Wang Y. M. Adv. Synth. Catal. 2020, 362, 506.
doi: 10.1002/adsc.v362.3 |
| [39] |
He M. X.; Mo Z. Y.; Wang Z. Q. Org. Lett. 2019, 22, 724.
doi: 10.1021/acs.orglett.9b04549 |
| [40] |
Javed M. I.; Brewer M. Org. Lett. 2007, 9, 1789.
doi: 10.1021/ol070515w |
| [41] |
Cheng N. L. Solvent Handbook, Ed.: Gu, N.-J., Chemical Industry Press, Beijing, 2015, p. 1112. (in Chinese)
|
|
( 程能林, 溶剂手册, 编辑: 顾南君, 化学工业出版社, 北京, 2015, p. 1112.)
|
|
| [42] |
Fang G. Y. US 20160060223, 2016.
|
| [43] |
Iwamoto R. T. Anal. Chem. 1959, 31, 955.
doi: 10.1021/ac60149a600 |
| [44] |
Mulina O. M. Org. Lett. 2022, 22, 1818.
doi: 10.1021/acs.orglett.0c00139 |
| [45] |
Mills A.; Holland C. J. Chem. Res., Synop. 1997, 368.
|
| [46] |
Furrow M. E.; Myers A. G. J. Am. Chem. Soc. 2004, 126, 12222.
doi: 10.1021/ja0459779 |
| [47] |
Liu W.; Twilton J.; Wei B. ACS Catal. 2021, 11, 2676.
doi: 10.1021/acscatal.1c00264 |
| [48] |
Ryzhakov A. V.; Andreev V. P. Russ. J. Org. Chem. 2016, 52, 513.
doi: 10.1134/S1070428016040059 |
| [1] | Tongyang Cao, Wei Li, Lijing Wang. Recent Progress in N-Iodosuccinimide (NIS)-Mediated Iodination Reactions [J]. Chinese Journal of Organic Chemistry, 2024, 44(2): 508-524. |
| [2] | Zhong Weihui;Zhang Yongmin. The samarium triiodide promoted the conjugate addition of aromatic amines to α,β-unsaturated nitriles(esters) [J]. Chin. J. Org. Chem., 2000, 20(5): 747-749. |
| [3] | Dai Zhifei;Peng Bixian. Synthesis and characterization of some new indocarbocyanines [J]. Chin. J. Org. Chem., 1998, 18(5): 414-418. |
| [4] | XU CUILIAN;CHEN ZHENCHU;YANG YUFENG. Synthesis of 5-alkyl-5-tosyloxyl-isopropylidene malonate [J]. Chin. J. Org. Chem., 1997, 17(5): 474-477. |
| [5] | ZHAO YINGDI. The application of hypervalent organoiodine compounds in organic synthesis [J]. Chin. J. Org. Chem., 1996, 16(1): 11-17. |
| [6] | Sun Yulong;Huang Zuen;Cai Ruifang;Wei Jingde;Chen Jian;Wang Jianwei;Yu Yong;Liu Erbao;Fang Daoyu. Alkylation of C~6~0, C~7~0 [J]. Chin. J. Org. Chem., 1993, 13(5): 549-551. |
| [7] | XIA CHIZHONG;ZHOU PEIWEN;DING JINGFAN;YAO ZIRONG;ZHAO MINLING. Synthesis of 1-methyl-2-phenyl-3-aryl imidazolidinium iodide and phenyl-substituted one carbon unit transfer reactions [J]. Chin. J. Org. Chem., 1991, 11(2): 154-159. |
| [8] | CHEN QINGYUN;WU JIANPING. Reaction of fluoroalkyl iodides with olefines in sodium trimethylsilyl oxide [J]. Chin. J. Org. Chem., 1990, 10(3): 274-277. |
| [9] | LIN RONGHUI;CHEN LIYA;ZHANG YONGMIN. Application of iodonium salts in organic synthesis [J]. Chin. J. Org. Chem., 1989, 9(3): 193-199. |
| [10] | ZHENG QINGUO;FU WENYI;CHEN BANGCHI;HUANG XIAN. Synthesis of α-iodo-α-methoxycarbonylmethylene triphenylarsorane and its reaction with aldehydes [J]. Chin. J. Org. Chem., 1988, 8(1): 61-64. |
| [11] | CHEN QINGYUN;QIU ZAIMING. Studies on fluoroalkylation and fluoroalkoxylation 17. A new method for preparation of 2-fluoroalkyl pyrrole [J]. Chin. J. Org. Chem., 1987, 7(1): 44-46. |
| [12] | CHEN QINGYUN;YANG ZHENYU. Studies on fluoroalkylation and fluoroalkoxylation 9. Platinum(o)-catalyzed addition reactions of fluoroalkyl iodides with olefins [J]. Chin. J. Org. Chem., 1986, 6(1): 41-43. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||