Chinese Journal of Organic Chemistry ›› 2024, Vol. 44 ›› Issue (12): 3678-3685.DOI: 10.6023/cjoc202405019 Previous Articles Next Articles
REVIEW
收稿日期:2024-05-15
修回日期:2024-08-09
发布日期:2024-09-10
作者简介:基金资助:
Yi-Yu Liu†, Yudong Hu†, Qing-Wei Zhang*( )
)
Received:2024-05-15
Revised:2024-08-09
Published:2024-09-10
Contact:
*E-mail:About author:Supported by:Share
Yi-Yu Liu, Yudong Hu, Qing-Wei Zhang. Advances in Mechanistic Studies of Metal-Catalyzed Hydrophosphinylation of Non-Activated Alkynes[J]. Chinese Journal of Organic Chemistry, 2024, 44(12): 3678-3685.
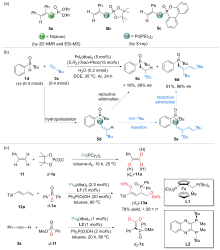

| [1] |
(a) Guo H.; Fan Y. C.; Sun Z.; Wu Y.; Kwon O. Chem. Rev. 2018, 118, 10049.
|
|
(b) Ni H.; Chan W.-L.; Lu Y. Chem. Rev. 2018, 118, 9344.
|
|
|
(c) Liu X., Wu Y., Zhang Q. Synlett 2022, 33, 301.
|
|
|
(d) Banerjee I.; Panda T. K. Org. Biomol. Chem. 2021, 19, 6571.
|
|
|
(e) Novas B. T.; Waterman R. ChemCatChem 2022, 14, e20220098.
|
|
| [2] |
Han L.-B.; Tanaka M. J. Am. Chem. Soc. 1996, 118, 1571.
|
| [3] |
Han L.-B.; Choi N.; Tanaka M. Organometallics 1996, 15, 3259.
|
| [4] |
Han L.-B.; Mirzaei F.; Zhao C.-Q.; Tanaka M. J. Am. Chem. Soc. 2000, 122, 5407.
|
| [5] |
Zhao C.-Q.; Han L.-B.; Tanaka M. Organometallics 2000, 19, 4196.
|
| [6] |
Dobashi N.; Fuse K.; Hoshino T.; Kanada J.; Kashiwabara T.; Kobata C.; Nune S. K.; Tanaka M. Tetrahedron Lett. 2007, 48, 4669.
|
| [7] |
Zhao C.-Q.; Han L.-B.; Goto M.; Tanaka M. Angew. Chem., Int. Ed. 2001, 40, 1929.
|
| [8] |
Han L.-B.; Zhao C.-Q.; Tanaka M. J. Org. Chem. 2001, 66, 5929.
pmid: 11511276 |
| [9] |
Han L.-B.; Zhang C.; Yazawa H.; Shimada S. J. Am. Chem. Soc. 2004, 126, 5080.
|
| [10] |
Khemchyan L. L.; Ivanova J. V.; Zalesskiy S. S.; Ananikov V. P.; Beletskaya I. P.; Starikova Z. A. Adv. Synth. Catal. 2014, 356, 771.
|
| [11] |
Zhang Y.-Q.; Han X.-Y.; Wu Y.; Qi P.-J.; Zhang Q.; Zhang Q.-W. Chem. Sci. 2022, 13, 4095.
|
| [12] |
Yang S.-N.; Sun S.-H.; Liu C.-H.; Min X.-T.; Wan B.; Ji D.-W.; Chen Q.-A. Chin. Chem. Lett. 2023, 34, 107914.
|
| [13] |
Qian D.-W.; Yang J.; Wang G.-W.; Yang S.-D. J. Org. Chem. 2023, 88, 3539.
|
| [14] |
Niu M.; Fu H.; Jiang Y.; Zhao Y. Chem. Commun. 2007, 272.
|
| [15] |
Liu L. L.; Wu Y.; Wang Z.; Zhu J.; Zhao Y. J. Org. Chem. 2014, 79, 6816.
doi: 10.1021/jo5007174 pmid: 24983135 |
| [16] |
Li J.; Gao Z.; Guo Y.; Liu H.; Zhao P.; Bi X.; Shi E.; Xiao J. RSC Adv. 2022, 12, 18889.
|
| [17] |
Wu Z.-H.; Cheng A.-Q.; Yuan M.; Zhao Y.-X.; Yang H.-L.; Wei L.-H.; Wang H.-Y.; Wang T.; Zhang Z.; Duan W.-L. Angew. Chem., Int. Ed. 2021, 60, 27241.
|
| [18] |
Nie S.-Z.; Davison R. T.; Dong V. M. J. Am. Chem. Soc. 2018, 140, 16450.
|
| [19] |
Yang Z.; Wang J. Angew. Chem., Int. Ed. 2021, 60, 27288.
|
| [20] |
Zhou J.; Meng L.; Lin S.; Cai B.; Wang J. Angew. Chem., Int. Ed. 2023, 62, e202303727.
|
| [21] |
Long J.; Li Y.; Zhao W.; Yin G. Chem. Sci. 2022, 13, 1390.
|
| [22] |
Han L.-B.; Zhao C.-Q.; Onozawa S.-y.; Goto M.; Tanaka M. J. Am. Chem. Soc. 2002, 124, 3842.
|
| [23] |
Dai Q.; Liu L.; Qian Y.; Li W.; Zhang J. Angew. Chem., Int. Ed. 2020, 59, 20645.
|
| [24] |
Yang Z.; Gu X.; Han L.-B.; Wang J. Chem. Sci. 2020, 11, 7451.
|
| [25] |
(a) Xu Q.; Han L.-B. J. Organomet. Chem. 2011, 696, 130.
|
|
(b) Ding K.; Su B. Eur. J. Org. Chem. 2024, 27, e202301160.
|
|
| [26] |
(a) Rosenberg L. ACS Catal. 2013, 3, 2845.
|
|
(b) Lecerclé D.; Sawicki M.; Taran F. Org. Lett. 2006, 8, 4283.
|
|
| [27] |
(a) Glueck D. S. Top. Organomet. Chem. 2010, 31, 65.
|
|
(b) Koshti V.; Gaikwad S.; Chikkali S. H. Coord. Chem. Rev. 2014, 265, 52.
|
|
|
(c) Bange C. A.; Waterman R. Chem. Eur. J. 2016, 22, 12598.
|
|
|
(d) Lau S.; Hood T. M.; Webster R. L. ACS Catal. 2022, 12, 10939.
|
|
| [28] |
Chen T.; Zhao C.-Q.; Han L.-B. J. Am. Chem. Soc. 2018, 140, 3139.
|
| [29] |
Liu S.-Y.; Li Z.-M.; Guo H.; Zhang J.-L. Eur. J. Org. Chem. 2023, 26, e202300804.
|
| [30] |
Chen T.; Han L.-B. Heteroat. Chem. 2018, 29, e21460.
|
| [31] |
Glueck D. S. J. Org. Chem. 2020, 85, 14276.
|
| [32] |
Han L.-B.; Hua R.; Tanaka M. Angew. Chem., Int. Ed. 1998, 37, 94.
|
| [33] |
Shen R.; Chen T.; Zhao Y.; Qiu R.; Zhou Y.; Yin S.; Wang X.; Goto M.; Han L.-B. J. Am. Chem. Soc. 2011, 133, 17037.
|
| [34] |
Jiang Y.-Y.; Fan X.; Li Y.; Ji G.-C.; Liu P.; Bi S. J. Org. Chem. 2022, 87, 14673.
|
| [35] |
Chen T.; Liu L.; Huang T.; Han L.-B. Chin. J. Org. Chem. 2019, 39, 2183 (in Chinese).
|
|
(陈铁桥, 刘龙, 黄添增, 韩立彪, 有机化学, 2019, 39, 2183.)
doi: 10.6023/cjoc201905001 |
|
| [36] |
(a) Gu J.; Cai C. Org. Biomol. Chem. 2017, 15, 4226.
|
|
(b) Chen X.; Chen X.; Li X.; Qu C.; Qu L.; Bi W.; Sun K.; Zhao Y. Tetrahedron 2017, 73, 2439.
|
|
|
(c) Liu W. Q.; Lei T.; Zhou S.; Yang X. L.; Li J.; Chen B.; Sivaguru J.; Tung C. H.; Wu L. Z. J. Am. Chem. Soc. 2019, 141, 13941.
|
|
| [37] |
(a) Wang H.; Li Y.; Tang Z.; Wang S.; Zhang H.; Cong H.; Lei A. ACS Catal. 2018, 8, 10599.
|
|
(b) Hou H.; Wang J.; Chen X.; Han Y.; Yu H.; Yan C.; Shi Y.; Zhu S. ChemCatChem 2022, 14, e202201073.
|
|
| [38] |
Yoo W.; Kobayashi S. Green Chem. 2013, 15, 1844.
|
| [39] |
(a) Jin S.; Sun S.; Yu J. T.; Cheng J. J. Org. Chem. 2019, 84, 11177.
|
|
(b) Hou H.; Xu Y.; Yang H.; Chen X.; Yan C.; Shi Y.; Zhu S. Org. Lett. 2020, 22, 1748.
|
|
| [40] |
Chen X.; Li X.; Chen X.; Qu L.; Chen J.; Sun K.; Liu Z.; Bi W.; Xia Y.; Wu H.; Zhao Y. Chem. Commun. 2015, 51, 3846.
|
| [41] |
(a) Liu J.; Xiao H. Z.; Fu Q.; Yu D. G. Chem. Synth. 2021, 1, 9.
|
|
(b) Yuan Y.; Darcel C. ChemCatChem 2024, e202400703.
|
| [1] | Yuetian Guo, Yongxin Pan, Lijun Tang. Progresses in Reactive Fluorescent Probes with Fused Aggregation- Induced Emission (AIE) and Excited State Intramolecular Proton Transfer (ESIPT) Structures [J]. Chinese Journal of Organic Chemistry, 2022, 42(6): 1640-1650. |
| [2] | Wang Ruixianga, Lai Xiaojinga, Qiu Guanyinsheng, Liu Jinbiao. Recent Advances in Reaction-Based Excited State Intramolecular Proton Transfer (ESIPT) Fluorescence Probe [J]. Chin. J. Org. Chem., 2019, 39(4): 952-960. |
| [3] | Liu, Yu, Tang, Yongxing, Luo, Yueyang, Zhu, Yeting, Yang, Jin, Wei, Xinyu, Liu, Xiong, Zhao, Yunhui. Synthesis and Application of Fluorescent Probe Containing Barbitone Unit [J]. Chinese Journal of Organic Chemistry, 2019, 39(10): 2980-2984. |
| [4] | Xu Dongdong, Shan Chunhui, Bai Ruopeng, Lan Yu. Mechanism of Alkaline Earth Metal Catalyzed Hydroboration of Carbodiimides: A Theoretical Study [J]. Chin. J. Org. Chem., 2017, 37(5): 1231-1236. |
| [5] | Shi Bochao, Fang Yewen, Zhang Li, Jin Xiaoping, Wu Yonghui, Fang Mei, Yang Yufei, Chen Chong. Advances in the Synthesis of α-Aryl Vinylphosphonates [J]. Chin. J. Org. Chem., 2016, 36(4): 673-686. |
| [6] | Du Lijuan, Wu Caihong, Gu Honghong, Li Juan. A Density Functional Theory Study of the Mechanism of Cu(II)-Catalyzed Synthesis of Pyrido[1,2-a]benzimidazoles [J]. Chin. J. Org. Chem., 2015, 35(8): 1726-1732. |
| [7] | Ma Tuo, Zhang Jin, Liu Longzhu, He Yun, Zhang Zunting. Synthesis and Characterization of a Visualization and Water-Soluble Fluorescent Probe for Al3+ [J]. Chin. J. Org. Chem., 2014, 34(9): 1780-1785. |
| [8] | Zhang Peng, Zhang Youming, Lin Qi, Yao Hong, Wei Taibao. Principle and the Research Progress of Fluorescent Chemosensors for Cations Recognition [J]. Chin. J. Org. Chem., 2014, 34(7): 1300-1321. |
| [9] | Yi Pinggui, Yang Xichun, Liu Jin, Hou Bo, Yu Xianyong, Li Xiaofang, Wang Zhaoxu, Zheng Baishu. Investigation of Excited-State Proton Transfer of 2-(3-Acetamido-2-pyridyl)benzimidazole in the Confined Nanocavity of Cucurbit[7]uril [J]. Chin. J. Org. Chem., 2013, 33(07): 1451-1456. |
| [10] | Hou Chuanjin, Liu Xiaoning, Xia Ying, Hu Xiangping. Progress on Unsymmetrical Hybrid Chiral Phosphine-phosphoramidite Ligands and Their Application in Asymmetric Catalytic Reactions [J]. Chin. J. Org. Chem., 2012, 32(12): 2239-2247. |
| [11] | CAI Meng-Jun, CHEN Jian-Ding. A New Ultrasonic Synthetic Method for Proton Transfer Compound of 2,6-Pyridinedicarboxylic Acid and 2,6-Pyridinediamine [J]. Chin. J. Org. Chem., 2010, 30(07): 1076-1079. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||