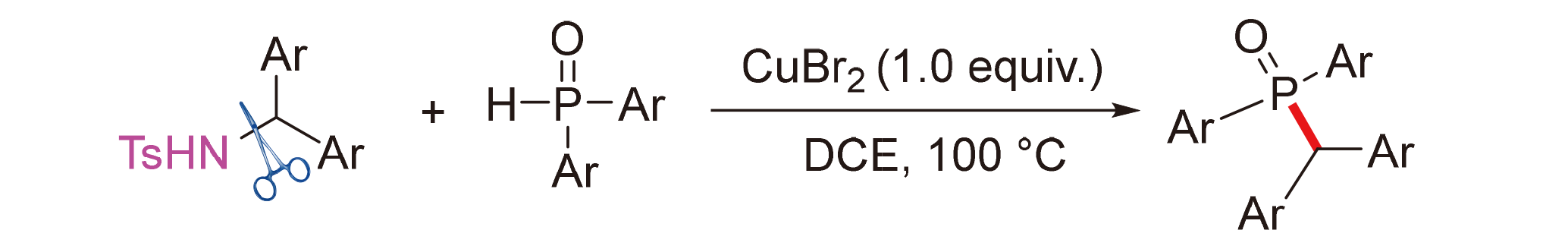-
-
 About the Cover:An organophosphoric acid catalyzed [3+3] cycliza-tion of 2-arylidene-indan-1,3-diones with enaminones has been established by Guo, Yu, Wan, Lu, Tan, and Shi on page 3727, affording a series of structurally diverse indenoquinolinedione derivatives in overall high yields. This [3+3] cyclization has some advantages such as mild reaction conditions, readily available catalyst and wide substrate range, which provides an efficient method for constructing biologically important 1,4-dihydro- pyridine motif.
About the Cover:An organophosphoric acid catalyzed [3+3] cycliza-tion of 2-arylidene-indan-1,3-diones with enaminones has been established by Guo, Yu, Wan, Lu, Tan, and Shi on page 3727, affording a series of structurally diverse indenoquinolinedione derivatives in overall high yields. This [3+3] cyclization has some advantages such as mild reaction conditions, readily available catalyst and wide substrate range, which provides an efficient method for constructing biologically important 1,4-dihydro- pyridine motif. -
 About the Cover:Phosphine-catalyzed [5+1] annulation of Morita- Baylis-Hillman (MBH) carbonates with 1,3-dicarbonyl compounds was reported by Ren, Shi, Tang and Guo on page 3739. The reaction worked well under mild reaction conditions. This is the first time that MBH carbonates functioned as all-carbon five-membered syn- thon in phosphine catalysis.
About the Cover:Phosphine-catalyzed [5+1] annulation of Morita- Baylis-Hillman (MBH) carbonates with 1,3-dicarbonyl compounds was reported by Ren, Shi, Tang and Guo on page 3739. The reaction worked well under mild reaction conditions. This is the first time that MBH carbonates functioned as all-carbon five-membered syn- thon in phosphine catalysis. -
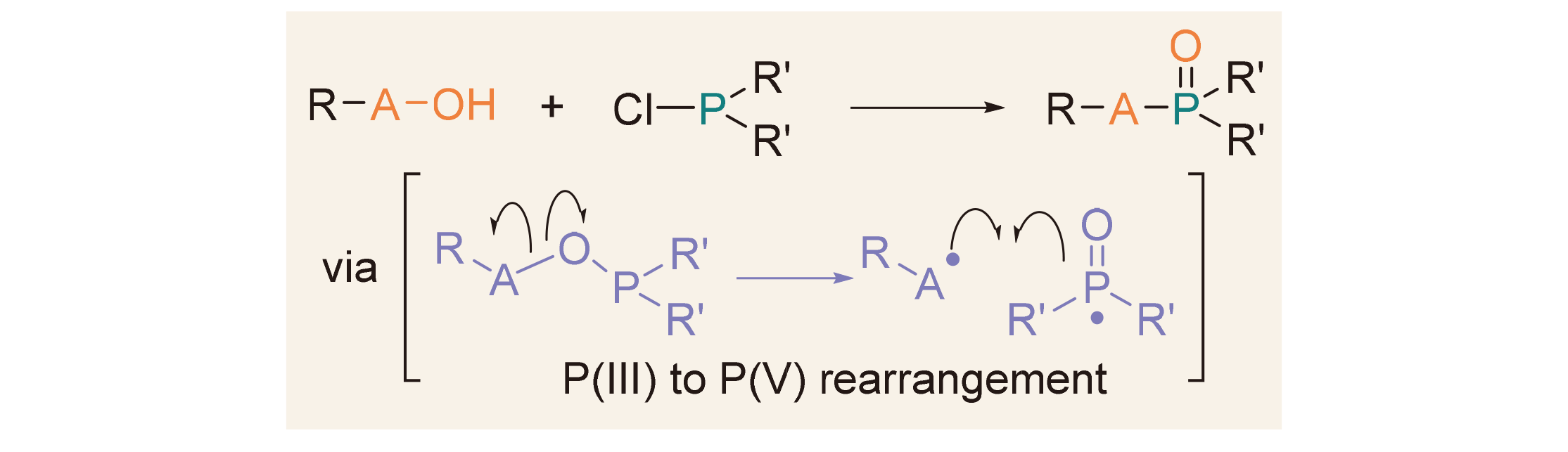
 About the Cover:Phosphine chlorides (R1R2PCl) and hydroxyl-containing compounds (RA—OH) can form a RA—O—PR1R2 intermediate, which undergoes A—O bond cleavage and A—P bond formation leading to P(III)→P(V) rear-rangement to form pentavalent organophosphorus com-pounds. The P(III)→P(V) rearrangement reactions of phosphine chlorides with different types of hydroxyl containing compounds for the synthesis of phosphine oxides, phosphonates, and phosphoric amides are re-viewed by Yang, Yi, Wu, Zhang, Bai, Li and Xu on page 3639.
About the Cover:Phosphine chlorides (R1R2PCl) and hydroxyl-containing compounds (RA—OH) can form a RA—O—PR1R2 intermediate, which undergoes A—O bond cleavage and A—P bond formation leading to P(III)→P(V) rear-rangement to form pentavalent organophosphorus com-pounds. The P(III)→P(V) rearrangement reactions of phosphine chlorides with different types of hydroxyl containing compounds for the synthesis of phosphine oxides, phosphonates, and phosphoric amides are re-viewed by Yang, Yi, Wu, Zhang, Bai, Li and Xu on page 3639. -
 About the Cover:The desulfurization reaction of alkenyl sulfonium salts with phosphine oxides to form C(sp2)—P bonds was rea- lized under room temperature. This method shows good functional group compatibility, offering a straightforward and efficient preparation route for terminal vinyl phosphine oxides. More details can be found in the article by An, Lv, Sun, Chen, Qu, and Yu on page 3747.
About the Cover:The desulfurization reaction of alkenyl sulfonium salts with phosphine oxides to form C(sp2)—P bonds was rea- lized under room temperature. This method shows good functional group compatibility, offering a straightforward and efficient preparation route for terminal vinyl phosphine oxides. More details can be found in the article by An, Lv, Sun, Chen, Qu, and Yu on page 3747.
-
-
Current Issue
REVIEW
ARTICLE
HIGHLIGHT