Chinese Journal of Organic Chemistry ›› 2025, Vol. 45 ›› Issue (1): 246-255.DOI: 10.6023/cjoc202406010 Previous Articles Next Articles
ARTICLES
王浩洋, 成琳, 曾星, 韩利民, 杜玉英*( ), 竺宁*(
), 竺宁*( )
)
收稿日期:2024-06-08
修回日期:2024-08-03
发布日期:2024-08-30
基金资助:
Haoyang Wang, Lin Cheng, Xing Zeng, Limin Han, Yuying Du( ), Ning Zhu(
), Ning Zhu( )
)
Received:2024-06-08
Revised:2024-08-03
Published:2024-08-30
Contact:
*E-mail: Supported by:Share
Haoyang Wang, Lin Cheng, Xing Zeng, Limin Han, Yuying Du, Ning Zhu. Functionalized Ionic Liquid [BMim]OH-Catalyzed the Synthesis of β-Ketosulfones and Sulfone Compounds between α-Halides and Sodium Sulfinate[J]. Chinese Journal of Organic Chemistry, 2025, 45(1): 246-255.
| Entry | 1a∶2a | [BMim]OHa/% | T/℃ | Solvent | Time/h | Yieldb/% |
|---|---|---|---|---|---|---|
| 1 | 1∶1 | — | r.t. | Ethanol (φ=50%) | 2 | 45 |
| 2 | 1∶1 | 20 | r.t. | Ethanol (φ=50%) | 2 | 68 |
| 3 | 1∶1.2 | 20 | r.t. | Ethanol (φ=50%) | 2 | 79 |
| 4 | 1∶1.5 | 20 | r.t. | Ethanol (φ=50%) | 2 | 94 (80c) |
| 5 | 1∶2 | 20 | r.t. | Ethanol (φ=50%) | 2 | 95 |
| 6 | 1∶1.5 | 5 | r.t. | Ethanol (φ=50%) | 2 | 79 |
| 7 | 1∶1.5 | 10 | r.t. | Ethanol (φ=50%) | 2 | 86 |
| 8 | 1∶1.5 | 15 | r.t. | Ethanol (φ=50%) | 2 | 89 |
| 9 | 1∶1.5 | 30 | r.t. | Ethanol (φ=50%) | 2 | 95 |
| 10 | 1∶1.5 | 20 | 60 | Ethanol (φ=50%) | 1.5 | 98 |
| 11 | 1∶1.5 | 20 | r.t. | H2O | 2 | 60 |
| 12 | 1∶1.5 | 20 | r.t. | Anhydrous ethanol | 2 | 65 |
| Entry | 1a∶2a | [BMim]OHa/% | T/℃ | Solvent | Time/h | Yieldb/% |
|---|---|---|---|---|---|---|
| 1 | 1∶1 | — | r.t. | Ethanol (φ=50%) | 2 | 45 |
| 2 | 1∶1 | 20 | r.t. | Ethanol (φ=50%) | 2 | 68 |
| 3 | 1∶1.2 | 20 | r.t. | Ethanol (φ=50%) | 2 | 79 |
| 4 | 1∶1.5 | 20 | r.t. | Ethanol (φ=50%) | 2 | 94 (80c) |
| 5 | 1∶2 | 20 | r.t. | Ethanol (φ=50%) | 2 | 95 |
| 6 | 1∶1.5 | 5 | r.t. | Ethanol (φ=50%) | 2 | 79 |
| 7 | 1∶1.5 | 10 | r.t. | Ethanol (φ=50%) | 2 | 86 |
| 8 | 1∶1.5 | 15 | r.t. | Ethanol (φ=50%) | 2 | 89 |
| 9 | 1∶1.5 | 30 | r.t. | Ethanol (φ=50%) | 2 | 95 |
| 10 | 1∶1.5 | 20 | 60 | Ethanol (φ=50%) | 1.5 | 98 |
| 11 | 1∶1.5 | 20 | r.t. | H2O | 2 | 60 |
| 12 | 1∶1.5 | 20 | r.t. | Anhydrous ethanol | 2 | 65 |
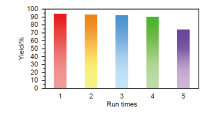
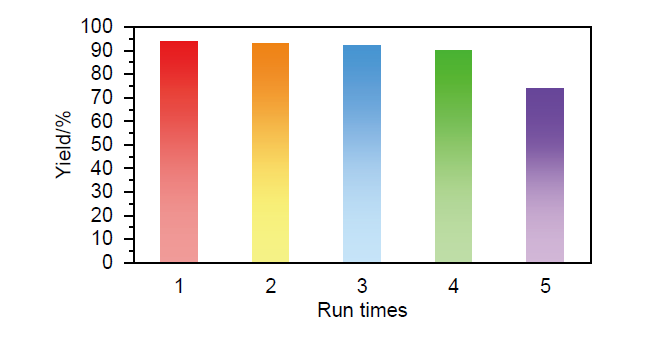
| Entry | Catalyst | Condition | Time | Yield/% | Ref. |
|---|---|---|---|---|---|
| 1 | KI | V(AcOH)∶V(DMSO)=1∶1, 80 ℃ | 12 h | 57 (3a) | [ |
| 2 | CuBr2 | DMSO, r.t. | 1 h | 85 (3a) | [ |
| 3 | Free Cat. | V(C2H5OH)∶V(H2O)=3∶1, 50 ℃ | 10 h | 65 (3m) | [ |
| 4 | Mn, I2 | DMA, r.t. | 24 h | 80 (3a) | [ |
| 5 | Cu25%/ZIF-8 | MeOH, r.t. | 12 h | 89 (3m) | [ |
| 6 | Et3N, I2 | H2O | 10 min | 85 (3a) | [ |
| 7 | PG(+)|St(-), KI, TBHP | MeOH, r.t. | 10 h | 82 (3a) | [ |
| 8 | [BMim]OH | V(EtOH)∶V(H2O)=1∶1, r.t. | 2 h | 94 (3a) | This work |
| Entry | Catalyst | Condition | Time | Yield/% | Ref. |
|---|---|---|---|---|---|
| 1 | KI | V(AcOH)∶V(DMSO)=1∶1, 80 ℃ | 12 h | 57 (3a) | [ |
| 2 | CuBr2 | DMSO, r.t. | 1 h | 85 (3a) | [ |
| 3 | Free Cat. | V(C2H5OH)∶V(H2O)=3∶1, 50 ℃ | 10 h | 65 (3m) | [ |
| 4 | Mn, I2 | DMA, r.t. | 24 h | 80 (3a) | [ |
| 5 | Cu25%/ZIF-8 | MeOH, r.t. | 12 h | 89 (3m) | [ |
| 6 | Et3N, I2 | H2O | 10 min | 85 (3a) | [ |
| 7 | PG(+)|St(-), KI, TBHP | MeOH, r.t. | 10 h | 82 (3a) | [ |
| 8 | [BMim]OH | V(EtOH)∶V(H2O)=1∶1, r.t. | 2 h | 94 (3a) | This work |
| [1] |
(a) Pal, T. K.; Dey, S.; Pathak, T. J. Org. Chem. 2011, 76, 3034.
|
|
(b) Xu, W. M.; Han, F.-F.; He, M.; Hu, D. Y.; He, J.; Yang, S.; Song, B. A. J. Agric. Food Chem. 2012, 60, 1036.
|
|
|
(c) Zhang, B.; Wassermann, A. M.; Vogt, M.; Bajorath, J. J. Chem. Inf. Model. 2012, 52, 3138.
|
|
|
(d) Consalvi, S.; Alfonso, S.; Di Capua, A.; Poce, G.; Pirolli, A.; Sabatino, M.; Ragno, R.; Anzini, M.; Sartini, S.; La Motta, C.; Di Cesare, M. L.; Ghelardini, C.; Biava, M. Bioorg. Med. Chem. 2015, 23, 810.
|
|
| [2] |
(a) Aranapakam, V.; Grosu, G. T.; Davis, J. M.; Hu, B.; Ellingboe, J.; Baker, J. L.; Skotnicki, J. S.; DiJoseph, J. F.; Sung, A.; Sharr, M. A.; Killar, L. M.; Walter, T.; Jin, G. X.; Cowling, R. J. Med. Chem. 2003, 46, 2361.
|
|
(b) Peng, H.; Cheng, Y.; Ni, N.; Li, M.; Choudhary, G.; Chou, H. T.; Lu, C. D.; Tai, P. C.; Wang, B. ChemMedChem 2009, 4, 1457.
|
|
|
(c) Abdel-Aziz, H. A.; Al-Rashood, K. A.; ElTahir, K. E. H.; Suddek, G. M. Eur. J. Med. Chem. 2014, 80, 416.
|
|
|
(d) Zheng, M.; Li, G.; Lu, H. Org. Lett. 2019, 21, 1216.
|
|
| [3] |
Yalavarthi, N. R.; Gundoju, N.; Bokam, R.; Ponnapalli, M. G. J. Chem. Sci. 2019, 131.
|
| [4] |
Han, F.; Su, B.; Song, P.; Wang, Y.; Jia, L.; Xun, S.; Hu, M.; Zou, L. Tetrahedron 2018, 74, 5908.
|
| [5] |
Yavari, I.; Shaabanzadeh, S. Org. Lett. 2020, 22, 464.
|
| [6] |
Xu, J.; Shen, C.; Qin, X.; Wu, J.; Zhang, P.; Liu, X. J. Org. Chem. 2021, 86, 3706.
|
| [7] |
Wang, Y. J.; Zhao, Y. H.; Cai, C. Q.; Wang, L. Y.; Gong, H. Org. Lett. 2021, 23, 8296.
|
| [8] |
Pampana, V. K. K.; Charpe, V. P.; Sagadevan, A.; Das, D. K.; Lin, C.-C.; Hwu, J. R.; Hwang, K. C. Green Chem. 2021, 23, 3569.
|
| [9] |
Fang, Y.; Xu, D. P.; Yu, Y. L.; Tang, R. M.; Dai, S.-S.; Wang, G. H. Eur. J. Org. Chem. 2022, 13, 1.
|
| [10] |
Song, Y. L.; Wang, X. C.; Huang, C. P.; Liang, F. B.; Yu, Z. C.; Chen, B. H. Chin. J. Org. Chem. 2013, 33, 1715 (in Chinese).
|
|
(宋彦磊, 王新承, 黄崇品, 梁凤兵, 毓志超, 陈标华, 有机化学, 2013, 33, 1715.)
|
|
| [11] |
(a) Lourenço, N. M. T.; Afonso, C. A. M. Tetrahedron 2003, 59, 789.
|
|
(b) Jorapur, Y. R.; Chi, D. Y. Bull. Korean Chem. Soc. 2006, 27, 345.
|
|
|
(c) Zhao, D. W.; Wu, Y. T.; Chen, T.-T.; Dai, L. Y.; Wang, Y.-Y. Chin. J. Org. Chem. 2013, 33, 1791 (in Chinese).
|
|
|
(赵东旺, 吴悦彤, 陈婷婷, 戴立益, 王媛媛, 有机化学, 2013, 33, 1791.)
|
|
| [12] |
(a) Hu, Y.; Chen, Z. C.; Le, Z. G.; Zheng, Q. G. J. Chem. Res. 2004, 4, 267.
|
|
(b) Li, J. X.; Yang, S. R.; Wu, W. Q.; Jiang, H. F. Eur. J. Org. Chem. 2018, 1284.
|
|
|
(c) Lai, Y. L.; Wu, L. Y.; Xiong, X.; Lan, Y. W.; Lin, Y.-Y.; Zhong, R. M.; Jiang, H. F.; Li, J. X. Green Synth. Catal.DOI: 10.1016/j.gresc.2024.02.003.
|
|
| [13] |
(a) Suryakiran, N.; Prabhakar, P.; Rajesh, K.; Suresh, V.; Venkateswarlu, Y. J. Mol. Catal. A: Chem. 2007, 270, 201.
|
|
(b) Hu, M.; Lin, Z. D.; Li, J. X.; Wu, W. Q.; Jiang, H. F. Green Chem. 2020, 22, 5584.
|
|
|
(c) Li, J. X.; He, D.; Lin, Z. D.; Cen, L. Y.; Wu, W. Q.; Jiang, H. F. Green Chem. 2022, 24, 1983.
|
|
| [14] |
Kumar, A.; Muthyala, M. K. Tetrahedron Lett. 2011, 52, 5368.
|
| [15] |
Moulton, R. US 20030094380, 2003.
|
| [16] |
Yuen, A. K. L.; Masters, A. F.; Maschmeyer, T. Catal. Today 2013, 200, 9.
|
| [17] |
Zhang, H. L.; Li, M. Y.; Wang, N. Appl. Chem. Ind. 2013, 42, 1866 (in Chinese).
|
|
(张海龙, 李梦耀, 王娜, 应用化工, 2013, 42, 1866.)
|
|
| [18] |
Song, Z.; Wang, H.; Xing, L. J. Solution Chem. 2009, 38, 1139.
|
| [19] |
Zhao, P.-P.; Hu, K.; Cai, P.; Cheng, G. Z. Univ. Chem. 2022, 37, 2208150 (in Chinese).
|
|
(赵苹苹, 胡锴, 蔡苹, 程功臻, 大学化学, 2022, 37, 2208150.)
|
|
| [20] |
Ye, M. F.; Cao, Y. Z.; Ding, R. H.; Lei, Z. P.; Jin, L.; Zhang, J. Chem. Ind. Times 2019, 33, 9 (in Chinese).
|
|
(叶明富, 曹云钟, 丁仁浩, 雷智平, 金玲, 张婧, 化工时刊, 2019, 33, 9.)
|
|
| [21] |
Liu, L.; Xiao, H.; Xiao, F. H.; Xie, Y. J.; Huang, H. W.; Deng, G. J. Chin. J. Org. Chem. 2021, 41, 4749 (in Chinese).
|
|
(刘丽, 肖洪, 肖福红, 谢艳军, 黄华文, 邓国军, 有机化学, 2021, 41, 4749.)
|
|
| [22] |
Xu, L.; Lv, L.-L.; Wang, X. S. Chin. J. Org. Chem. 2023, 43, 3644 (in Chinese).
|
|
(许力, 吕兰兰, 王香善, 有机化学, 2023, 43, 3644.)
|
|
| [23] |
Gulizabair, A. M.D. Dissertation, Xinjiang Normal University, Xinjiang, 2021 (in Chinese).
|
|
(古丽扎拜尔•阿不力皮孜, 硕士论文, 新疆师范大学, 新疆, 2021.)
|
|
| [24] |
Chen, Z.; Guo, K.; Chen, R. S.; Gu, C.; Zhou, H. T.; Zhu, Y. G. Chin. J. Org. Chem. 2018, 38, 963 (in Chinese).
|
|
(陈震, 郭康, 陈荣顺, 顾晨, 周华婷, 朱映光, 有机化学, 2018, 38, 963.)
|
|
| [25] |
He, Y.; Yin, H. G.; Zeng, Z. W.; Zeng, H. T. Shandong Chem. Ind. 2022, 51, 8 (in Chinese).
|
|
(何燕, 尹红果, 曾智文, 曾慧婷, 山东化工, 2022, 51, 8.)
|
|
| [26] |
(a) Liu, C. R.; Ding, L. H.; Guo, G.; Liu, W.-W. Eur. J. Org. Chem. 2016, 2016, 910.
|
|
(b) Suryakiran, N.; Reddy, T. S.; Ashalatha, K.; Lakshman, M.; Venkateswarlu, Y. Tetrahedron. Lett. 2006, 47, 3853.
|
|
| [27] |
(a) Rawat, V. S.; Reddy, P. L. M.; Sreedhar, B. RSC. Adv. 2014, 4, 5165.
|
|
(b) Tang, X.; Huang, L.; Xu, Y.; Yang, J.; Wu, W.; Jiang, H. Angew. Chem., Int. Ed. 2014, 53, 4205.
|
|
| [28] |
(a) Yang, J.; Li, H. Q.; Li, M. H.; Peng, J.-J.; Gu, Y. L. Adv. Synth. Catal. 2012, 354, 688.
|
|
(b) Chang, M. Y.; Cheng, Y. C.; Lu, Y. J. Org. Lett. 2014, 16, 6252.
|
|
| [29] |
(a) Fu, J.; Li, Q. Z.; Li, M. P.; Du, Z. Y. ChemistrySelect 2020, 5, 2985.
|
|
(b) Chen, X. Y.; Lu, S. X.; Zheng, Y.-Y.; Wang, J. G.; Yang, L.; Sun, P. Adv. Synth. Catal. 2022, 364, 1305.
|
|
| [30] |
(a) Wagh, G. D.; Autade, S. B.; Kulkarni, R. V.; Akamanchi, K. G. New J. Chem. 2020, 44, 10554.
|
|
(b) Truitt, P.; Stead, R.; Long, L. M.; Middleton, W. J. J. Am. Chem. Soc. 1949, 71, 3511.
|
|
| [31] |
Jiang, H.; Cheng, Y.; Zhang, Y.; Yu, S. Eur. J. Org. Chem. 2013, 2013, 5485.
|
| [32] |
(a) Xiong, Y. H.; Weng, J.; Lu, G. Adv. Synth. Catal. 2018, 360, 1611.
|
|
(b) Lu, Q.; Zhang, J.; Zhao, G.; Qi, Y.; Wang, H.; Lei, A. J. Am. Chem. Soc. 2013, 135, 11481.
|
|
| [33] |
Tsui, G. C.; Glenadel, Q.; Lau, C.; Lautens, M. Org. Lett. 2011, 13, 208.
|
| [34] |
Yuan, Z. Y.; Zhang, S.; Teng, F.; Jin, X. F.; Sheng, W. B.; Gui, Q. W. ChemistrySelect 2022, 7, e202103355.
|
| [35] |
(a) Reddy, R. J.; Kumar, J.-J.; Kumari, A. H. Eur. J. Org. Chem. 2019, 23, 3771.
|
|
(b) Jeyakumar, K.; Chand, D. K. Tetrahedron Lett. 2006, 47, 4573.
|
|
|
(c) Wang, Z.; Jiang, J.; Pang, S.; Zhou, Y.; Guan, C.; Gao, Y.; Li, J.; Yang, Y.; Qiu, W.; Jiang, C. C. Environ. Sci. Technol. 2018, 52, 11276.
|
|
| [36] |
Wildeman, J.; Van Leusen, A. M. Synthesis 1979, 1979, 733.
|
| [37] |
Karimi, B.; Khorasani, M. ACS. Catal. 2013, 3, 1657.
|
| [38] |
Zhang, F. X.; Zhou, F. X.; Yin, S. H.; Long, B.; Deng, G. J.; Ali, A.; Song, T. Appl. Catal., B 2023, 337, 123004.
|
| [39] |
Onanong, V.; Khanchyd, M.; Praewpan, K.; Chutima, K.; Chutima, J. Bioorg. Med. Chem. Lett. 2022, 63, 128652
|
| [1] | Jiabin Shen, Chao Shen, Pengfei Zhang. Synthesis of Nafimidone Derivatives by Visible-Light-Induced α-C—H Functionalization of Carbonyl [J]. Chinese Journal of Organic Chemistry, 2025, 45(2): 677-685. |
| [2] | Enze Lin, Bijie Li. Recent Progress in Metal-Catalyzed Asymmetric Hydroarylation of Internal Alkenes Through C—H Cleavage [J]. Chinese Journal of Organic Chemistry, 2025, 45(2): 546-558. |
| [3] | Chaowei Zhang, Bingbin Xu, Wenlong Liu, Jing Zhao, Weiliang Duan. Palladium-Catalyzed Asymmetric C—H Activation for the Synthesis of Planar Chiral Ferrocene Sulfonamides [J]. Chinese Journal of Organic Chemistry, 2025, 45(2): 707-716. |
| [4] | Xun Tian, Guogang Deng, Xiaodong Yang. Progress on Cobalt-Catalyzed C(sp2)—H Activation for the Construction of Nitrogen-Containing Benzo Heterocycles [J]. Chinese Journal of Organic Chemistry, 2025, 45(2): 655-667. |
| [5] | Xiaoqin Wang, Sheng Xu, Yuanyuan Ping, Wangqing Kong. Selective Functionalization of C(sp3)—H Bonds via Photoredox/ Nickel Dual Catalysis [J]. Chinese Journal of Organic Chemistry, 2025, 45(2): 383-422. |
| [6] | Mingshun Mei, Yanghui Zhang. Palladium-Catalyzed Intermolecular Functionalization of Unactivated Methylene C(sp3)—H Bonds [J]. Chinese Journal of Organic Chemistry, 2025, 45(2): 620-640. |
| [7] | Yongmei Li, Liangbo Sun, Kun Xu, Chengchu Zeng. Recent Advances toward Electro- and Electrophotochemical 2,3-Dichloro-5,6-dicyano-1,4-benzoquinone (DDQ)-Catalyzed C—H/C—F Bonds Functionalization [J]. Chinese Journal of Organic Chemistry, 2025, 45(2): 668-676. |
| [8] | Yu Zou, Weicong Guo, Jun Wang. Application of Planar Chiral Cyclopentadienyl Rhodium Catalysts without Chiral Substituents in Asymmetric C—H Activation [J]. Chinese Journal of Organic Chemistry, 2025, 45(2): 466-476. |
| [9] | Chen-Hui Yuan, Lei Jiao. Chiral Ligands for Palladium-Catalyzed Coordination-Assisted Enantioselective C(sp3)—H Functionalization Reactions [J]. Chinese Journal of Organic Chemistry, 2025, 45(2): 602-619. |
| [10] | Fan Yang, Xiaomeng Fan, Xuejing Yao, Ruijie Mi, Songjie Yu, Xingwei Li, Jian Xiao. Rhodium(III)-Catalyzed Annulative Coupling between Sulfoxonium Ylides and Diazo Compounds [J]. Chinese Journal of Organic Chemistry, 2025, 45(1): 331-342. |
| [11] | Zhenru Huang, Guoshun Jin, Tianyu Chen, Bin Feng, Xinkang Shi, Minfang Chen, Lusheng Hua, Qing Xu. Efficient Construction of Quinoxaline Derivatives by Cesium Hydroxide-Catalyzed Mild Aerobic Annulation Reaction [J]. Chinese Journal of Organic Chemistry, 2024, 44(9): 2933-2942. |
| [12] | Yushen Gao, Yuanyuan Gao, An'an Zhang, Lu Li, Weizhi Geng, Fenghua Zhang, Fei Li, Lantao Liu. BF3•OEt2 Mediated Intramolecular Cyclization of 2-Alkynylanilines Approach to 3-Sulfenylindoles [J]. Chinese Journal of Organic Chemistry, 2024, 44(9): 2785-2795. |
| [13] | Jiayan Du, Juntao Liu, Guixia Liu, Zheng Huang. Cobalt-Catalyzed Regio- and Stereoselective Isomerization of Terminal Alkenes to trans-2-Alkenes [J]. Chinese Journal of Organic Chemistry, 2024, 44(9): 2889-2897. |
| [14] | Liangming Xuan, Wei Zhao, Rundong Fan, Qiongjiao Yan, Wei Wang, Fen'er Chen. Recent Advances of the Catalysis Systems in the α-C(sp3)—H Functionalization of Glycine Derivatives [J]. Chinese Journal of Organic Chemistry, 2024, 44(9): 2700-2721. |
| [15] | Yanhua Fu, Chang Xu, Chao Zhang, Yisha Wang, Gaofeng Feng. Visible-Light Induced Iron-Catalyzed Hydroxymethylation of N-Heterocycles [J]. Chinese Journal of Organic Chemistry, 2024, 44(7): 2265-2273. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||