Chinese Journal of Organic Chemistry ›› 2021, Vol. 41 ›› Issue (9): 3571-3577.DOI: 10.6023/cjoc202104025 Previous Articles Next Articles
ARTICLES
收稿日期:2021-04-12
修回日期:2021-05-25
发布日期:2021-07-12
通讯作者:
林旭锋, 杨妲
基金资助:
Huan Liu, Xufeng Lin( ), Da Yang(
), Da Yang( )
)
Received:2021-04-12
Revised:2021-05-25
Published:2021-07-12
Contact:
Xufeng Lin, Da Yang
Supported by:Share
Huan Liu, Xufeng Lin, Da Yang. Novel Ir-Complexes for Hydroformylation of Olefins with H2O as the Hydrogen Source[J]. Chinese Journal of Organic Chemistry, 2021, 41(9): 3571-3577.
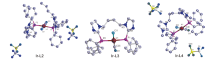

| Ir-complex | Selected bond length/nm | Bond angle/(o) | ||
|---|---|---|---|---|
| Ir—P1 | Ir—P2 | Ir—CCO | P1—Ir—P2 | |
| Ir-L2 | 0.23145(19) | 0.23173(19) | 0.18509(12) | 176.65(7) |
| Ir-L3 | 0.23177(10) | 0.23301(10) | 0.18629(56) | 173.85(3) |
| Ir-L4 | 0.23147(31) | 0.23152(30) | 0.18309(11) | 173.79(11) |
| Ir(CO)(PPh3)2Cl | 0.23309(10) | — | 0.17911(13) | 180.00(-) |
| Ir-complex | Selected bond length/nm | Bond angle/(o) | ||
|---|---|---|---|---|
| Ir—P1 | Ir—P2 | Ir—CCO | P1—Ir—P2 | |
| Ir-L2 | 0.23145(19) | 0.23173(19) | 0.18509(12) | 176.65(7) |
| Ir-L3 | 0.23177(10) | 0.23301(10) | 0.18629(56) | 173.85(3) |
| Ir-L4 | 0.23147(31) | 0.23152(30) | 0.18309(11) | 173.79(11) |
| Ir(CO)(PPh3)2Cl | 0.23309(10) | — | 0.17911(13) | 180.00(-) |
| Entry | Cat. | Conv.b/% | Sel.b/% | Yield/% | L/Bb | ||
|---|---|---|---|---|---|---|---|
| Aldehyde | Isomer | hexane | |||||
| 1 | Ir-L1 | 72 | 99 | <1 | <1 | 72 | 77/23 |
| 2 | Ir-L2 | 87 | 99 | <1 | <1 | 87 | 78/22 |
| 3 | Ir-L3 | 76 | 99 | <1 | <1 | 76 | 76/24 |
| 4 | Ir-L4 | 94 | 99 | <1 | <1 | 94 | 82/18 |
| 5c | Ir-PPh3 | 57 | 99 | <1 | <1 | 87 | 73/27 |
| 6 | — | 12 | 99 | <1 | <1 | 12 | 72/28 |
| 7d | Ir-L4 | 93 | 75 | 2 | 23 | 70 | 80/20 |
| Entry | Cat. | Conv.b/% | Sel.b/% | Yield/% | L/Bb | ||
|---|---|---|---|---|---|---|---|
| Aldehyde | Isomer | hexane | |||||
| 1 | Ir-L1 | 72 | 99 | <1 | <1 | 72 | 77/23 |
| 2 | Ir-L2 | 87 | 99 | <1 | <1 | 87 | 78/22 |
| 3 | Ir-L3 | 76 | 99 | <1 | <1 | 76 | 76/24 |
| 4 | Ir-L4 | 94 | 99 | <1 | <1 | 94 | 82/18 |
| 5c | Ir-PPh3 | 57 | 99 | <1 | <1 | 87 | 73/27 |
| 6 | — | 12 | 99 | <1 | <1 | 12 | 72/28 |
| 7d | Ir-L4 | 93 | 75 | 2 | 23 | 70 | 80/20 |
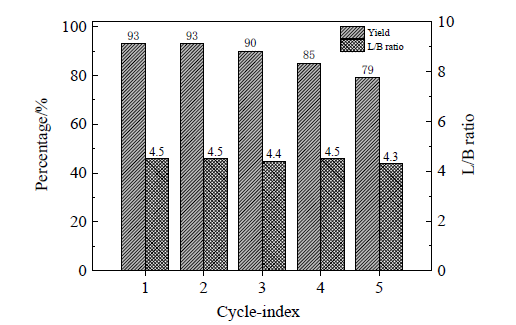
| [1] |
Kubis, C.; Selent, D.; Sawall, M.; Ludwig, R.; Neymeyr, K.; Baumann, W.; Franke, R.; Börner, A. Chem.-Eur. J. 2012, 18, 8780.
|
| [2] |
Franke, R.; Selent, D.; Börner, A. Chem. Rev. 2012, 112, 5675.
doi: 10.1021/cr3001803 |
| [3] |
Rein, C.; Demel, P.; Outten, R. A.; Netscher, T.; Breit, B. Angew. Chem. Int. Ed. 2007, 46, 8670.
doi: 10.1002/anie.v46:45 |
| [4] |
Kohlpaintner, C. W.; Fischer, R. W.; Cornils, B. Appl. Catal. A- Gen. 2001, 221, 219.
doi: 10.1016/S0926-860X(01)00791-8 |
| [5] |
Cornils, B. Org. Proc. Res. Dev. 1998, 2, 121.
|
| [6] |
Li, Y.-Q.; Wang, P.; Zhang, H.; Zhao, X.-L.; Lu, Y.; Popović, Z.; Liu, Y. J. Mol. Catal. A-Chem. 2015, 402, 37.
doi: 10.1016/j.molcata.2015.02.016 |
| [7] |
van der Veen, L. A.; Kamer, P. C. J.; van Leeuwen, P. W. N. M. Angew. Chem. 1999, 111, 349.
doi: 10.1002/(ISSN)1521-3757 |
| [8] |
van der Veen, L. A.; Kamer, P. C. J.; van Leeuwen, P. W. N. M. Organometallics 1999, 18, 4765
doi: 10.1021/om990523j |
| [9] |
Frey, G. D. J. Organomet. Chem. 2014, 754, 5.
|
| [10] |
Behr, A.; Kämper, A.; Nickel, M.; Franke, R. Appl. Catal. A-Gen. 2015, 505, 243.
doi: 10.1016/j.apcata.2015.08.001 |
| [11] |
Piras, I.; Jennerjahn, R.; Jackstell, R.; Spannenberg, A.; Franke, R.; Beller, M. Angew. Chem. Int. Ed. 2011, 50, 280.
doi: 10.1002/anie.v50.1 |
| [12] |
Zhang, H.; Li, Y.-Q.; Wang, P.; Lu, Y.; Zhao, X.-L.; Liu, Y. J. Mol. Catal. A-Chem. 2016, 411, 337.
doi: 10.1016/j.molcata.2015.11.005 |
| [13] |
Behr, A.; Kämper, A.; Kuhlmann, R.; Vorholt, A. J.; Franke, R. Catal. Sci. Technol. 2016, 6, 208.
doi: 10.1039/C5CY01018G |
| [14] |
Biosca, M.; Magre, M.; Coll, M.; Pàmies, O.; Diéguez, M. Adv. Synth. Catal. 2017, 359, 2801.
doi: 10.1002/adsc.v359.16 |
| [15] |
Margarita, C.; Andersson, P. G. J. Am. Chem. Soc. 2017, 139, 1346.
doi: 10.1021/jacs.6b10690 |
| [16] |
Sawano, T.; Lin, Z.; Boures, D.; An, B.; Wang, C.; Lin, W. J. Am. Chem. Soc. 2016, 138, 9783.
doi: 10.1021/jacs.6b06239 |
| [17] |
Mazloomi, Z.; Pretorius, R.; Pàmies, O.; Albrecht, M.; Diéguez, M. Inorg. Chem. 2017, 56, 11282.
doi: 10.1021/acs.inorgchem.7b01707 pmid: 28849942 |
| [18] |
Liu, H.; Yang, D.; Yao, Y.; Xu, Y.; Shang, H.; Lin, X. Mol. Catal. 2020, 485, 110843.
|
| [19] |
Liu, H.; Liu, L.; Guo, W.-D.; Lu, Y.; Zhao, X.-L.; Liu, Y. J. Catal. 2019, 373, 215.
|
| [20] |
Vandenberg, D. M.; Suzuki, T. M.; Ford, P. C. J. Organomet. Chem. 1984, 272, 309.
|
| [21] |
Lin, J.; Wang, A.; Qiao, B.; Liu, X.; Yang, X.; Wang, X.; Liang, J.; Li, J.; Liu, J.; Zhang, T. J. Am. Chem. Soc. 2013, 135, 15314.
doi: 10.1021/ja408574m |
| [22] |
Liu, H.; Yang, D.; Wang, D.-L.; Wang, P.; Lu, Y.; Giang, V.-T.; Liu, Y. Chem. Commun. 2018, 54, 7979.
doi: 10.1039/C8CC03431A |
| [23] |
Chikkali, S. H.; van der Vlugt, J. I.; Reek, J. N. H. Coord. Chem. Rev. 2014, 262, 1.
doi: 10.1016/j.ccr.2013.10.024 |
| [24] |
Grushin, V. V. Chem. Rev. 2004, 104, 1629.
doi: 10.1021/cr030026j |
| [25] |
Aguado-Ullate, S.; Baker, J. A.; González-González, V.; Müller, C.; Hirst, J. D.; Carbó, J. J. Catal. Sci. Technol. 2014, 4, 979.
doi: 10.1039/C3CY00956D |
| [26] |
Tricas, H.; Diebolt, O.; van Leeuwen, P. W. N. M. J. Catal. 2013, 298, 198.
|
| [27] |
Diebolt, O.; Tricas, H.; Freixa, Z.; van Leeuwen, P. W. N. M. ACS Catal. 2013, 3, 128.
doi: 10.1021/cs300470u |
| [28] |
Wu, X.-F.; Fang, X.; Wu, L.; Jackstell, R.; Neumann, H.; Beller, M. Acc. Chem. Res. 2014, 47, 1041.
doi: 10.1021/ar400222k |
| [29] |
Li, Y.-Q.; Wang, P.; Liu, H.; Lu, Y.; Zhao, X.-L.; Liu, Y. Green Chem. 2016, 18, 1798.
doi: 10.1039/C5GC02127H |
| [30] |
Šebesta, R.; Kmentová, I.; Toma, Š. Green Chem. 2008, 10, 484.
doi: 10.1039/b801456f |
| [31] |
Yang, D.; Liu, H.; Wang, D.-L.; Luo, Z.; Lu, Y.; Xia, F.; Liu, Y. Green Chem. 2018, 20, 2588.
doi: 10.1039/C8GC00754C |
| [32] |
Ni, B.; Headley, A. D. Chem.-Eur. J. 2010, 16, 4426.
|
| [33] |
Liu, H.; Wang, D.-L.; Chen, X.; Lu, Y.; Zhao, X.-L.; Liu, Y. Green Chem. 2017, 19, 1109.
doi: 10.1039/C6GC03096C |
| [34] |
Jeulin, S.; Duprat de Paule, S.; Ratovelomanana-Vidal, V.; Genêt, J.-P.; Champion, N.; Dellis, P. Angew. Chem. Int. Ed. 2004, 43, 320.
|
| [35] |
Suárez, A.; Méndez-Rojas, M. A.; Pizzano, A. Organometallics 2002, 21, 4611.
doi: 10.1021/om020140c |
| [36] |
Rosales, M.; Durán, J. A.; González, Á.; Pacheco, I.; Sánchez- Delgado, R. A. J. Mol. Catal. A-Chem. 2007, 270, 250.
doi: 10.1016/j.molcata.2007.01.045 |
| [37] |
Huo, C.; Chan, T. H. Chem. Soc. Rev. 2010, 39, 2977.
doi: 10.1039/b914497h |
| [38] |
Okano, T.; Kobayashi, T.; Konishi, H.; Kui, J. Bull. Chem. Soc. Jpn. 1981, 84, 3799.
|
| [39] |
Rosales, M.; Durán, J. A.; González, Á.; Pacheco, I.; Sánchez- Delgado, R. A. J. Mol. Catal. A-Chem. 2007, 270, 250.
doi: 10.1016/j.molcata.2007.01.045 |
| [40] |
Liu, L.; Guo, H.; Yang, S.-Q,; Chen, X.-C.; Lu, Y.; Liu, Y.; Xia, F. J. Catal. 2020, 385, 183.
|
| [1] | Peng Wang, Da Yang, Huan Liu. Recent Advances on Carbonylation of 1,3-Dienes [J]. Chinese Journal of Organic Chemistry, 2021, 41(9): 3379-3389. |
| [2] | Zong Lingbo, Chen Jianbin, Ren Xinyi, Zhang Guoying, Jia Xiaofei. Progress in Application of Organic Polymers Supported Rhodium Catalysts in Hydroformylation [J]. Chinese Journal of Organic Chemistry, 2020, 40(8): 2308-2321. |
| [3] | Li Shuailong, Li Zhuangxing, You Cai, Lü Hui, Zhang Xumu. Recent Advances in Asymmetric Hydroformylation [J]. Chin. J. Org. Chem., 2019, 39(6): 1568-1582. |
| [4] | Jia Xiaofei, Ren Xinyi, Wang Zheng, Xia Chungu, Ding Kuiling. Pyrrolyl-Based Phosphoramidite/Rh Catalyzed Asymmetric Hydroformylation of 1,1-Disubstituted Olefins [J]. Chin. J. Org. Chem., 2019, 39(1): 207-214. |
| [5] | Xing Aiping, Tian Mi, Wang Lailai. Novel Chiral C3-Symmetric Monophosphite Ligands: Synthsis and Catalytic Performance in Asymmetric Hydroformylation and 1,4-Conjugate Addition [J]. Chin. J. Org. Chem., 2016, 36(12): 2912-2919. |
| [6] | Jia Xiaofei, Wang Zheng, Xia Chungu, Ding Kuiling. Recent Advances in Rh-Catalyzed Asymmetric Hydroformylation of Olefins [J]. Chin. J. Org. Chem., 2013, 33(07): 1369-1381. |
| [7] | Hou Chuanjin, Liu Xiaoning, Xia Ying, Hu Xiangping. Progress on Unsymmetrical Hybrid Chiral Phosphine-phosphoramidite Ligands and Their Application in Asymmetric Catalytic Reactions [J]. Chin. J. Org. Chem., 2012, 32(12): 2239-2247. |
| [8] | DONG Jian-Xun, FENG Xiao-Yan, WU Hua-Wen, SHI Yu-Kun, DING Zhen-Zhen, HU Xiao-Jing, HUANG Wei-Ping. Progress in the Directionally Selected Addition of Conjugated Diole-fine [J]. Chin. J. Org. Chem., 2011, 31(9): 1357-1368. |
| [9] | HU Feng, GUO Kuai, DONG Jian-Xun, DIAO Qi-Hua, HUANG Wei-Beng. Cobalt-Based Catalyst for Hydroformylation of Cyclohexene [J]. Chin. J. Org. Chem., 2010, 30(04): 528-533. |
| [10] | GONG Yong-Hua, XUE Hao-Ran, XIE Zai-Ku, JIN,Zhao-Sheng, YANG Fan, HE Ming-Yuan. Hydroformylation of 1-Butene in [Bmim]BF4-H2O [J]. Chinese Journal of Organic Chemistry, 2004, 24(9): 1108-1110. |
| [11] | YANG PEIYI;FU HONGXIANG;LUO YUZHONG. Catalytic activity of hydroformylation of tetranuclearcobalt carbonyl cluster supported on polymer of crown ether. [J]. Chin. J. Org. Chem., 1984, 4(2): 107-110. |
| [12] | WANG YUNPU;WU NAN;ZHANG AIMIN;LUO YUZHONG;FU HONGXIANG. Supported metal cluster iv. Cobalt carbonyl cluster supported on polystyrene [J]. Chin. J. Org. Chem., 1983, 3(5): 341-344. |
| [13] | ZHAI WEIXU;LI DAGANG;MA YUYUAN;ZHAO ZHUANYUN. Study on the Synthesis and catalytic activities of mixed-metal clusters 1. syntheses and catalytic activities of Pt-Co carbonyl clusters [J]. Chin. J. Org. Chem., 1983, 3(3): 180-187. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||