Chinese Journal of Organic Chemistry ›› 2022, Vol. 42 ›› Issue (9): 2640-2658.DOI: 10.6023/cjoc202203034 Previous Articles Next Articles
REVIEWS
收稿日期:2022-03-18
修回日期:2022-05-19
发布日期:2022-05-26
通讯作者:
贾雪锋
基金资助:Received:2022-03-18
Revised:2022-05-19
Published:2022-05-26
Contact:
Xuefeng Jia
Supported by:Share
Xuefeng Jia, Xiangjuan Tong. Recent Progress on Chan-Lam Coupling Reactions Catalyzed by Copper(II) Complexes[J]. Chinese Journal of Organic Chemistry, 2022, 42(9): 2640-2658.
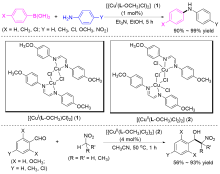
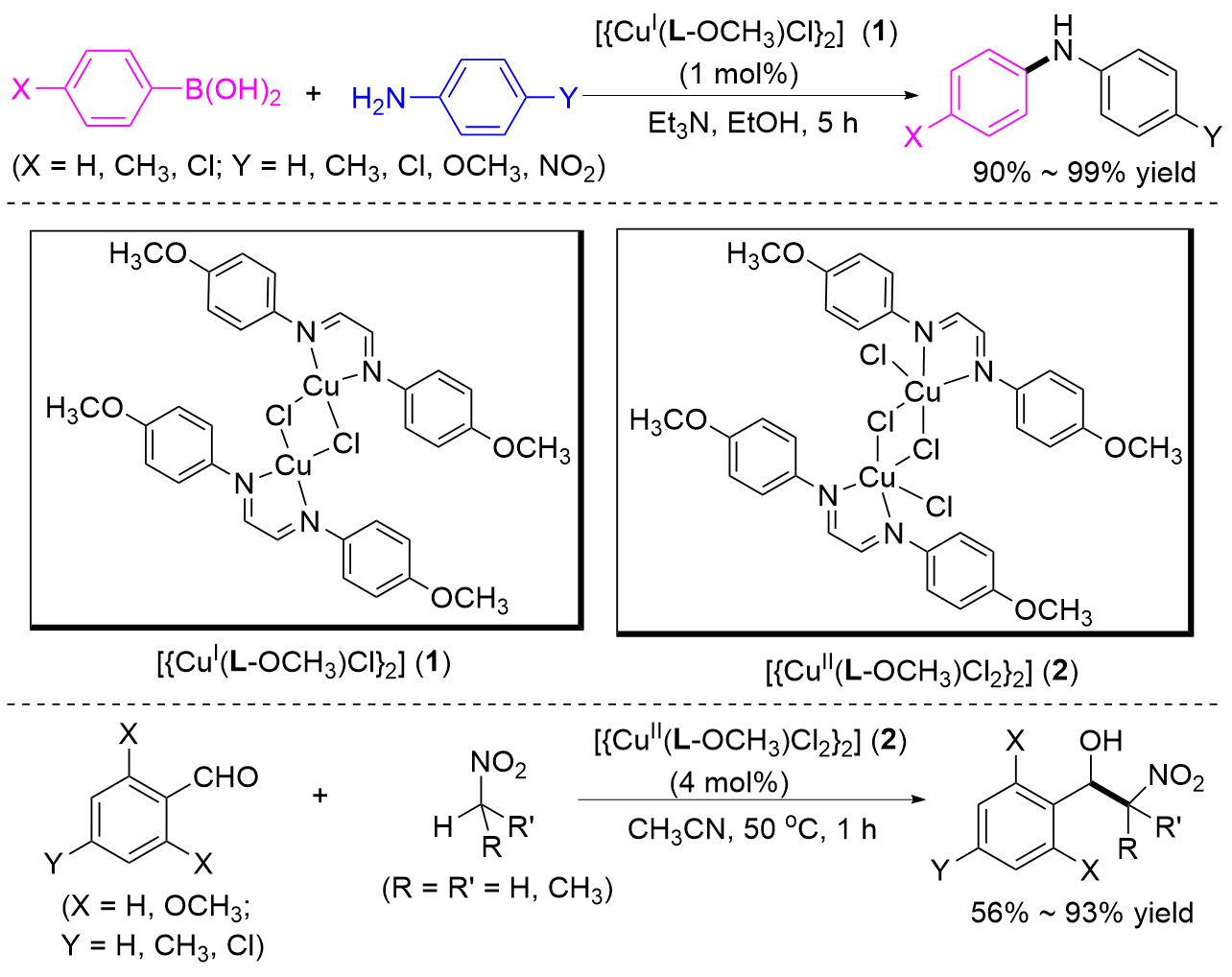
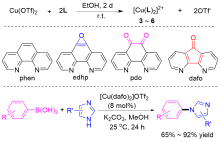
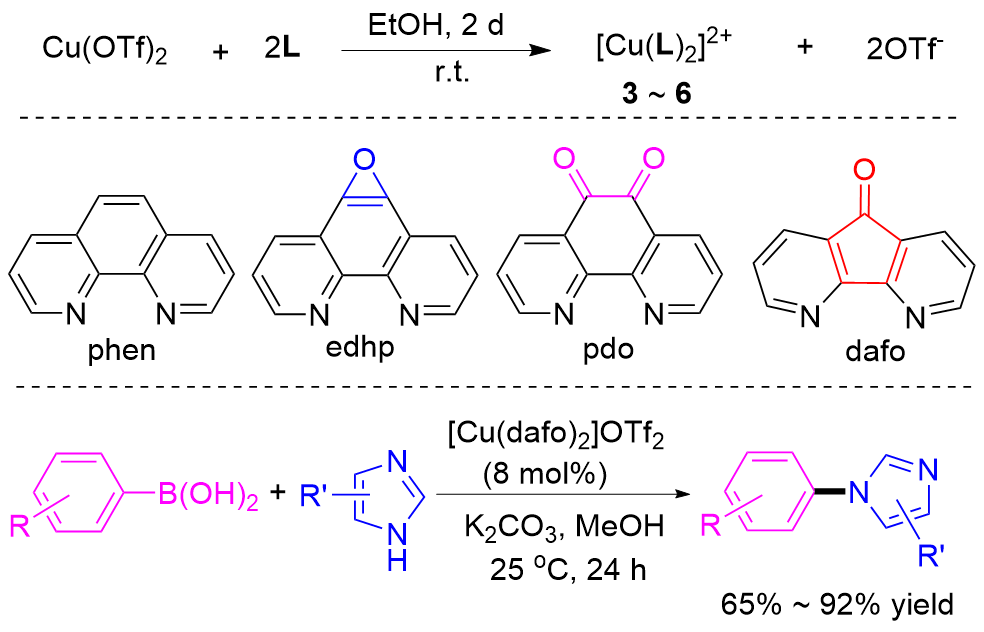
| [1] |
Chan, D. M. T.; Monaco, K. L.; Wang, R.-P.; Winters, M. P. Tetrahedron Lett. 1998, 39, 2933.
doi: 10.1016/S0040-4039(98)00503-6 |
| [2] |
Evans, D. A.; Katz, J. L.; West, T. R. Tetrahedron Lett. 1998, 39, 2937.
doi: 10.1016/S0040-4039(98)00502-4 |
| [3] |
Lam, P. Y. S.; Clark, C. G.; Saubern, S.; Adams, J.; Winters, M. P.; Chan, D. M. T.; Combs, A. Tetrahedron Lett. 1998, 39, 2941.
|
| [4] |
(a) Qiao, J. X.; Lam, P. Y. S. Synthesis 2011, 829.
|
|
(b) Vijayan, A.; Rao, D. N.; Radhakrishnan, K. V.; Lam, P. Y. S.; Das, P. Synthesis 2021, 53, 805.
doi: 10.1055/s-0040-1705971 |
|
| [5] |
West, M. J.; Fyfe, J. W. B.; Vantourout, J. C.; Watson, A. J. B. Chem. Rev. 2019, 119, 12491.
doi: 10.1021/acs.chemrev.9b00491 |
| [6] |
Fernandes, R. A.; Bhowmik, A.; Yadav, S. S. Org. Biomol. Chem. 2020, 18, 9583.
doi: 10.1039/d0ob02035d pmid: 33206103 |
| [7] |
Doyle, M. G. J.; Lundgren, R. J. Chem. Commun. 2021, 57, 2724.
doi: 10.1039/D1CC00213A |
| [8] |
Duan, X.; Liu, N.; Wang, J.; Ma, J. Chin. J. Org. Chem. 2019, 39, 661. (in Chinese)
|
|
(段希焱, 刘宁, 王佳, 马军营, 有机化学, 2019, 39, 661.)
doi: 10.6023/cjoc201808015 |
|
| [9] |
Ma, X. P.; Liu, F. P.; Mo, D. L. Chin. J. Org. Chem. 2017, 37, 1069. (in Chinese)
doi: 10.6023/cjoc201702001 |
|
(马小盼, 刘凤萍, 莫冬亮, 有机化学, 2017, 37, 1069.)
doi: 10.6023/cjoc201702001 |
|
| [10] |
Chen, J.-Q.; Li, J.-Q.; Dong, Z.-B. Adv. Synth. Catal. 2020, 362, 3311.
doi: 10.1002/adsc.202000495 |
| [11] |
Park, K. C.; Fouani, L.; Jansson, P. J.; Wooi, D.; Sahni, S.; Lane, D. J. R.; Palanimuthu, D.; Lok, H. C.; Kovaevi, Z.; Huang, M. L. H.; Kalinowski, D. S.; Richardson, D. R.; Metallomics 2016, 8, 874.
doi: 10.1039/C6MT00105J |
| [12] |
(a) Kahn, O. Acc. Chem. Res. 2000, 33, 647.
doi: 10.1021/ar9703138 |
|
(b) Li, W.; Cong, S.; Jiu, J.; Nagao, S.; Suganuma, K. J. Mater. Chem. C 2016, 4, 8802.
doi: 10.1039/C6TC02914K |
|
| [13] |
Merkle, A. C.; Lehnert, N. Dalton Trans. 2012, 41, 3355.
doi: 10.1039/c1dt11049g pmid: 21918782 |
| [14] |
Paterson, B. M.; Donnelly, P. S. Chem. Soc. Rev. 2011, 40, 3005.
doi: 10.1039/c0cs00215a pmid: 21409228 |
| [15] |
Pintauer, T.; Matyjaszewski, K. Chem. Soc. Rev. 2008, 37, 1087.
doi: 10.1039/b714578k |
| [16] |
(a) Zhang, M.-T.; Chen, Z.; Kang, P.; Meyer, T. J. J. Am. Chem. Soc. 2013, 135, 2048.
doi: 10.1021/ja3097515 |
|
(b) Su, X.-J.; Gao, M.; Jiao, L.; Liao, R.-Z.; Siegbahn, P. E. M.; Cheng, J.-P.; Zhang, M.-T. Angew. Chem., Int. Ed. 2015, 54, 4909.
doi: 10.1002/anie.201411625 |
|
|
(c) Liu, C.; Lei, H.; Zhang, Z.; Chen, F.; Cao, R. Chem. Commun. 2017, 53, 3189.
doi: 10.1039/C6CC09206C |
|
|
(d) Fisher, K. J.; Materna, K. L.; Mercado, B. Q.; Crabtree, R. H.; Brudvig, G. W. ACS Catal. 2017, 7, 3384.
doi: 10.1021/acscatal.7b00494 |
|
|
(e) Hu, Q.-Q.; Su, X.-J.; Zhang, M.-T. Inorg. Chem. 2018, 57, 10481.
doi: 10.1021/acs.inorgchem.8b01173 |
|
| [17] |
Collman, J. P.; Zhong, M. Org. Lett. 2000, 2, 1233.
pmid: 10810715 |
| [18] |
Collman, J. P.; Zhong, M.; Zhang, C.; Costanzo, S. J. Org. Chem. 2001, 66, 7892.
pmid: 11701055 |
| [19] |
Berkel, S. S.; Hoogenband, A.; Terpstra, J. W.; Tromp, M.; Leeuwena, P. W. N. M.; Strijdonck, G. P. F. Tetrahedron Lett. 2004, 45, 7659.
doi: 10.1016/j.tetlet.2004.08.094 |
| [20] |
Onaka, T.; Umemoto, H.; Miki, Y.; Nakamura, A.; Maegawa, T. J. Org. Chem. 2014, 79, 6703.
doi: 10.1021/jo500862t |
| [21] |
Roy, S.; Sarma, M. J.; Kashyap, B.; Phukan, P. Chem. Commun. 2016, 52, 1170.
doi: 10.1039/C5CC04619J |
| [22] |
Mukherjee, A.; Basu1, S.; Bhattacharya, S. Inorg. Chim. Acta 2020, 500, 119228.
doi: 10.1016/j.ica.2019.119228 |
| [23] |
Cope, J. D.; Valle, H. U.; Hall, R. S.; Riley, K. M.; Goel, E.; Biswas, S.; Hendrich, M. P.; Wipf, D. O.; Stokes, S. L.; Emerson, J. P. Eur. J. Inorg. Chem. 2020, 1278.
|
| [24] |
Gogoi, A.; Sarmah, G.; Dewan, A.; Bora, U. Tetrahedron Lett. 2014, 55, 31.
doi: 10.1016/j.tetlet.2013.10.084 |
| [25] |
Jia, X. F.; Peng, P.; Cui, J.; Xin, N. N.; Huang, X. Q. Asian J. Org. Chem. 2018, 7, 1093.
doi: 10.1002/ajoc.201800153 |
| [26] |
Jia, X. F.; Peng, P. Org. Biomol. Chem. 2018, 16, 8984.
doi: 10.1039/C8OB02254B |
| [27] |
Duparc, V. H.; Schaper, F. Organometallics, 2017, 36, 3053.
doi: 10.1021/acs.organomet.7b00397 |
| [28] |
Duparc, V. H.; Schaper, F. Dalton Trans. 2017, 46, 12766.
doi: 10.1039/C7DT02260C |
| [29] |
Duparc, V. H.; Bano, G. L.; Schaper, F. ACS Catal. 2018, 8, 7308.
doi: 10.1021/acscatal.8b01881 |
| [30] |
Duparc, V. H.; Dimeck, C.; Schaper, F. Can. J. Chem. 2019, 97, 178.
doi: 10.1139/cjc-2018-0402 |
| [31] |
Duparc, V. H.; Thouvenin, A.; Schaper, F. Can. J. Chem. 2020, 98, 502.
doi: 10.1139/cjc-2020-0003 |
| [32] |
Belokon, Y.; Akatyev, N. V.; Il'in, M. M.; Il'in, M. M.; Peregudova, S. M.; Peregudov, A. S.; Buyanovskaya, A. G.; Kudryavtsev, K. R.; Dubovik, A. S.; Grinberg, V. Y.; Orlov, V. N.; Pavlov, A. A.; Novikov, V. V.; Volkov, I. O. ChemCatChem 2020, 12, 3010.
doi: 10.1002/cctc.202000212 |
| [33] |
Hopkinson, M. N.; Richter, C.; Schedler, M.; Glorius, F. Nature 2014, 510, 485.
doi: 10.1038/nature13384 |
| [34] |
(a) Scott, N. M.; Nolan, S. P. Eur. J. Inorg. Chem. 2005, 1815.
|
|
(b) Yang, L.; Guo, Q.; Xiao, Y.; Mao, P. Chin. J. Org. Chem. 2015, 35, 1834. (in Chinese)
doi: 10.6023/cjoc201503040 |
|
|
(杨亮茹, 郭旗, 肖咏梅, 毛璞, 有机化学, 2015, 35, 1834.)
doi: 10.6023/cjoc201503040 |
|
| [35] |
Chung, L.-H.; Chan, S.-C.; Lee, W.-C.; Wong, C.-Y. Inorg. Chem. 2012, 51, 8693.
doi: 10.1021/ic202726g |
| [36] |
Oehninger, L.; Rubbiani, R.; Ott, I. Dalton Trans. 2013, 42, 3269.
doi: 10.1039/c2dt32617e pmid: 23223752 |
| [37] |
(a) Peris, E.; Crabtree, R. H. Coord. Chem. Rev. 2004, 248, 2239.
doi: 10.1016/j.ccr.2004.04.014 pmid: 19588961 |
|
(b) Díez-González, S.; Marion, N.; Nolan, S. P. Chem. Rev. 2009, 109, 3612.
doi: 10.1021/cr900074m pmid: 19588961 |
|
| [38] |
Lin, I. J. B.; Vasam, C. S. Coord. Chem. Rev. 2007, 251, 642.
doi: 10.1016/j.ccr.2006.09.004 |
| [39] |
(a) Wang, A.; Xiao, Y.; Zhou, Y.; Xu, J.; Liu, H. Chin. J. Org. Chem. 2017, 37, 2590. (in Chinese)
|
|
(王翱, 肖永龙, 周宇, 徐进宜, 柳红, 有机化学, 2017, 37, 2590.)
doi: 10.6023/cjoc201702041 |
|
|
(b) Qu, M.; He, J. Chin. J. Org. Chem. 2011, 31, 1388. (in Chinese)
|
|
|
(屈孟男, 何金梅, 有机化学, 2011, 31, 1388.)
|
|
|
(c) Li, T.; Jin, Z.; Chi, Y. R. Sci. China: Chem. 2022, 65, 210. (in Chinese)
doi: 10.1007/s11426-021-1133-5 |
|
|
(李婷婷, 金智超, 池永贵, 中国科学: 化学, 2022, 65, 210.)
|
|
| [40] |
Liu, B.; Liu, B.; Zhou, Y. B.; Chen, W. Z. Organometallics 2010, 29, 1457.
doi: 10.1021/om100009u |
| [41] |
Cope, J. D.; Sheridan, P. E.; Galloway, C. J.; Awoyemi, R. F.; Stokes, S. L.; Emerson, J. P. Organometallics 2020, 39, 4457.
doi: 10.1021/acs.organomet.0c00552 |
| [42] |
Astakhov, G. S.; Levitsky, M. M.; Bantreil, X.; Lamaty, F.; Khrustalev, V. N.; Zubavichus, Y. V.; Dorovatovskii, P. V.; Shubina, E. S.; Bilyachenko, A. N. J. Organomet. Chem. 2019, 906, 121022.
doi: 10.1016/j.jorganchem.2019.121022 |
| [43] |
Kulakova, A. N.; Khrustalev, V. N.; Zubavichus, Y. V.; Shul'pina, L. S.; Shubina, E. S.; Levitsky, M. M.; Ikonnikov, N. S.; Bilyachenko, A. N.; Kozlov, Y. N.; Shul'pin, G. B. Catalysts 2019, 9, 154
doi: 10.3390/catal9020154 |
| [44] |
Dronova, M. S.; Bilyachenko, A. N.; Yalymov, A. I.; Kozlov, Y. N.; Shul'pina, L. S.; Korlyukov, A. A.; Arkhipov, D. E.; Levitsky, M. M.; Shubina, E. S.; Shul'pin, G. B. Dalton Trans. 2014, 43, 872.
doi: 10.1039/c3dt52508b pmid: 24154485 |
| [45] |
Yoo, A. J.; Tsukamoto, T.; Kobayashi, S. Angew. Chem., Int. Ed. 2015, 54, 6587.
doi: 10.1002/anie.201500074 |
| [46] |
Bi, H. Y.; Li, C. J.; Wei, C.; Liang, C.; Mo, D. L. Green Chem. 2020, 22, 5815.
doi: 10.1039/D0GC01514H |
| [47] |
Zhao, J.; Huang, B. Q.; Zhu, B. C.; Ma, X. P.; Mo, D. L. Adv. Synth. Catal. 2021, 363, 4575.
doi: 10.1002/adsc.202100448 |
| [48] |
Wang, C.; Zhang, H.; Wells, L. A.; Liu, T.; Meng, T. T.; Liu, Q. C.; Walsh, P. J.; Kozlowski, M. C.; Jia, T. Z. Nat. Commun. 2021, 12, 932.
doi: 10.1038/s41467-021-21156-w |
| [49] |
Chiang, G. C. H.; Olsson, T. Org. Lett. 2004, 6, 3079.
pmid: 15330592 |
| [50] |
Kantam, M. L.; Venkanna, G. T.; Sridhar, C.; Sreedhar, B.; Choudary, B. M. J. Org. Chem. 2006, 71, 9522.
doi: 10.1021/jo0614036 |
| [51] |
Mostafalu, R.; Kaboudin, B.; Kazemia, F.; Yokomatsu, T. RSC Adv. 2014, 4, 49273.
doi: 10.1039/C4RA08137D |
| [52] |
Puthiaraj, P.; Pitchumani, K. Chem.-Eur. J. 2014, 20, 8761.
doi: 10.1002/chem.201402365 |
| [53] |
Anuradha; Kumari, S.; Pathak, D. D. Tetrahedron Lett. 2015, 56, 4135.
doi: 10.1016/j.tetlet.2015.05.049 |
| [54] |
Kumar, A.; Layek, S.; Agrahari, B.; Kujur, S.; Pathak, D. D. ChemistrySelect 2019, 4, 1337.
doi: 10.1002/slct.201803113 |
| [55] |
Devarajan, N.; Suresh, P. ChemCatChem 2016, 8, 2953.
doi: 10.1002/cctc.201600480 |
| [56] |
Lin, Y.; Cai, M. Z.; Fang, Z. Q.; Zhao, H. Tetrahedron 2016, 72, 3335.
doi: 10.1016/j.tet.2016.04.063 |
| [57] |
Khosravi, A.; Mokhtari, J.; Naimi-Jamal, M. R.; Tahmasebia, S.; Panahi, L. RSC Adv. 2017, 7, 46022.
doi: 10.1039/C7RA09772G |
| [58] |
Han, Y.; Zhang, M.; Zhang, Y.-Q.; Zhang, Z.-H. Green Chem. 2018, 20, 4891.
doi: 10.1039/C8GC02611D |
| [59] |
Liu, H, S.; Yu, Z. Q.; Sun, Z. C.; Wang, Y.; Liu, Y. Y.; Wang, A. J. Chem. J. Chin. Univ. 2019, 41, 1091. (in Chinese)
|
|
(刘恒烁, 遇治权, 孙志超, 王瑶, 刘颖雅, 王安杰, 高等学校化学学报, 2019, 41, 1091.)
|
|
| [60] |
Jamwal, B.; Kaur, M.; Sharma, H.; Khajuria, C.; Paul, S.; Clark, J. H. New J. Chem. 2019, 43, 4919.
doi: 10.1039/C8NJ05050C |
| [61] |
Vibhute, S. P.; Mhaldar, P. M.; Gaikwad, D. S.; Shejwal, R. V.; Pore, D. M. Monatsh. Chem. 2020, 151, 87.
|
| [62] |
Sarmah, M.; Dewan, A.; Boruah, P. K.; Das, M. R.; Bora, U. Appl. Organomet. Chem. 2020, 34, e5554.
|
| [63] |
Huang, X. Q.; Qi, Y. Q.; Gu, Y. X.; Gong, S. W.; Shen, G. D.; Li, Q.; Li, J. K. Dalton Trans. 2020, 49, 10970.
doi: 10.1039/D0DT02162H |
| [64] |
Sharma, S.; Kaur, M.; Sharma, C.; Choudhary, A.; Paul, S. ACS Omega 2021, 6, 19529.
doi: 10.1021/acsomega.1c01830 |
| [65] |
Di, J.-Q.; Zhang, M.; Chen, Y.-X.; Wang, J.-X.; Geng, S.-S.; Tang, J.-Q.; Zhang, Z.-H. Green Chem. 2021, 23, 1041.
doi: 10.1039/D0GC03400B |
| [66] |
Zhang, C. L.; Zhu, H.; Gang, K. Y.; Tao, M. L.; Ma, N.; Zhang, W. Q. React. Funct. Polym. 2021, 160, 104831.
doi: 10.1016/j.reactfunctpolym.2021.104831 |
| [67] |
Lam, P. Y. S.; Bonne, D.; Vincent, G.; Clark, C. G.; Combs, A. P. Tetrahedron Lett. 2003, 44, 1691.
|
| [68] |
(a) King, A. E.; Brunold, T. C.; Stahl, S. S. J. Am. Chem. Soc. 2009, 131, 5044.
doi: 10.1021/ja9006657 |
|
(b) King, A. E.; Ryland, B. L.; Brunold, T. C.; Stahl, S. S. Organometallics 2012, 31, 7948.
doi: 10.1021/om300586p |
|
| [69] |
(a) Vantourout, J. C.; Miras, H. N.; Isidro-Llobet, A.; Sproules, S.; Watson, A. J. B. J. Am. Chem. Soc. 2017, 139, 4769.
doi: 10.1021/jacs.6b12800 pmid: 28266843 |
|
(b) Vantourout, J. C.; Li, L.; Bendito-Moll, E.; Chabbra, S.; Arrington, K.; Bode, B. E.; Isidro-Llobet, A.; Kowalski, J. A.; Nilson, M. G.; Wheelhouse, K. M. P.; Woodard, J. L.; Xie, S.; Leitch, D. C.; Watson, A. J. B. ACS Catal. 2018, 8, 9560.
doi: 10.1021/acscatal.8b03238 pmid: 28266843 |
| [1] | Rui Bai, Xujuan Liu, Wenyu Luo, Shanshan Liu, Linyu Jiao. Research Progress of Chan-Lam Coupling Reaction in Heterogeneous Catalysis [J]. Chinese Journal of Organic Chemistry, 2022, 42(8): 2342-2354. |
| [2] | Wang Lin, Yang Lili, Ou Yunfu, Xu Shihai, Lin Qifu, Yang Dingqiao. Platinum-Catalyzed syn-Stereocontrolled Ring-Opening of Oxabicyclic Alkenes with Arylsulfonyl Hydrazides [J]. Chinese Journal of Organic Chemistry, 2020, 40(12): 4228-4236. |
| [3] | Ouyang Yao, Xu Xiuhua, Qing Fengling. Oxidative Coupling Reactions of Arylboronic Acids and Fluoroform-Derived AgCF3 [J]. Chinese Journal of Organic Chemistry, 2020, 40(10): 3426-3430. |
| [4] | Duan Xiyan, Liu Ning, Wang Jia, Ma Junying. Progress in Copper-Catalyzed Chan-Lam Coupling of N-Compounds [J]. Chin. J. Org. Chem., 2019, 39(3): 661-667. |
| [5] | Li Hengchao, Zhao Ling, Liu Yan, Zhang Xia, Li Wangbing, Jing Linhai, Huang Jin, Wang Wei. An Efficient Palladium Nanoparticles Catalytic System for Suzuki Coupling Reactions [J]. Chinese Journal of Organic Chemistry, 2019, 39(11): 3207-3214. |
| [6] | Xu Pei, Wang Shun-Yi, Fang Yi, Ji Shun-Jun. Research Progress on the Reaction of Carbon Dioxide with Nucleophiles [J]. Chin. J. Org. Chem., 2018, 38(7): 1626-1637. |
| [7] | Liu Boqu, Yan Zhongfei, Quan Zhengjun. Palladium/Copper(I) Acetate-Promoted Desulfurative Coupling of Pyrimidine Thioether with Alkynes or Arylboronic Acids [J]. Chin. J. Org. Chem., 2018, 38(11): 3032-3038. |
| [8] | Yan Xiaohui, Li Jiarong, Zhang Qi, Shi Daxin. Synthesis of Nitroarenes under Microwave [J]. Chin. J. Org. Chem., 2017, 37(6): 1450-1455. |
| [9] | Deng Yingying, Yang Wen, Yang Xin, Yang Dingqiao. Progress in Iridium-Catalyzed Asymmetric Allylic Substitution Reactions with Allylic Esters [J]. Chin. J. Org. Chem., 2017, 37(12): 3039-3059. |
| [10] | Chu Xu, Chang Honghong, Gao Wenchao, Wei Wenlong, Li Xing. Research Progress in the Ring-Opening Reactions of Aziridines by Carbon Nucleophiles [J]. Chin. J. Org. Chem., 2017, 37(10): 2569-2589. |
| [11] | Li Zhen, Duan Weiliang. Recent Advances in the Asymmetric Conjugate Addition Reactions of Phosphorus Nucleophiles to Electron-Deficient Alkenes [J]. Chin. J. Org. Chem., 2016, 36(8): 1805-1813. |
| [12] | Wu Ruihua, Yang Wen, Cheng Guo, Li Yue, Yang Dingqiao. Progress in Transition Metal-Catalyzed Asymmetric Ring-Opening Reactions of Oxa(Aza)bicyclic Alkenes with Carbanion Nucleophiles [J]. Chin. J. Org. Chem., 2016, 36(10): 2368-2379. |
| [13] | Xu Qing, Jia Xiaojuan, Li Xiaohui, Sun Qing, Zhou Yongbo, Yin Shuangfeng, Han Libiao. Copper-Catalyzed Aerobic Oxidative C-P Coupling of Arylboronic Acids and Diethyl Phosphite under Air [J]. Chin. J. Org. Chem., 2014, 34(7): 1340-1346. |
| [14] | Luo Renshi, Liao Jianhua, Zhang Jian. Progress in the Asymmetric Metal-Catalyzed 1,2-Addition Reactions of Arylboronic Acids with Ketones [J]. Chin. J. Org. Chem., 2013, 33(11): 2298-2309. |
| [15] | Yang Minghua, Pei Ji, Yan Guobing, Weng Qiuyue. Base-Free Cu(II)-Catalyzed Coupling Reaction of Arylboronic Acids and Thiols [J]. Chin. J. Org. Chem., 2013, 33(02): 343-347. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
