Chinese Journal of Organic Chemistry ›› 2022, Vol. 42 ›› Issue (9): 2877-2887.DOI: 10.6023/cjoc202204021 Previous Articles Next Articles
ARTICLES
黄兴, 张昌浩, 邓浩, 沈庆坤, 郭红艳, 全哲山, 李志勇*( ), 金莉莉*(
), 金莉莉*( )
)
收稿日期:2022-04-07
修回日期:2022-05-25
发布日期:2022-06-08
通讯作者:
李志勇, 金莉莉
作者简介:基金资助:
Xing Huang, Changhao Zhang, Hao Deng, Qingkun Shen, Hongyan Guo, Zheshan Quan, Zhiyong Li( ), Lili Jin(
), Lili Jin( )
)
Received:2022-04-07
Revised:2022-05-25
Published:2022-06-08
Contact:
Zhiyong Li, Lili Jin
About author:Supported by:Share
Xing Huang, Changhao Zhang, Hao Deng, Qingkun Shen, Hongyan Guo, Zheshan Quan, Zhiyong Li, Lili Jin. Synthesis and Antitumor Activity of Hederagenin Derivatives[J]. Chinese Journal of Organic Chemistry, 2022, 42(9): 2877-2887.
| Compd. | R | Growth inhibition/% | |||||
|---|---|---|---|---|---|---|---|
| HCT116 | SW620 | BEL7402 | HepG2 | MCF-7 | A549 | ||
| He | — | 26.21 | 24.22 | 31.19 | 19.69 | 32.43 | NA |
| A1 | 4-H | 46.13 | 24.42 | 34.24 | 39.81 | NA | NA |
| A2 | 4-Cl | NA | 28.11 | 30.67 | NA | 17.38 | NA |
| A3 | 3,4-Cl2 | 31.23 | 20.86 | NA | 56.07 | NA | 32.54 |
| A4 | 4-OCH3 | NA | NA | NA | NA | 36.86 | NA |
| A5 | 4-NO2 | 90.48 | 27.22 | 39.52 | 53.79 | 11.00 | 44.60 |
| B1 | 4-H | 29.55 | NA | 17.24 | 32.26 | 37.48 | 25.71 |
| B2 | 4-F | 28.77 | NA | 53.10 | 34.31 | 34.81 | NA |
| B3 | 4-Cl | NA | 12.81 | 27.10 | 31.26 | 44.29 | 23.73 |
| B4 | 3,4-Cl2 | NA | 17.94 | NA | NA | 45.76 | 55.56 |
| B5 | 4-OCH3 | NA | 44.08 | NA | 46.43 | 48.24 | 41.75 |
| B6 | 3,4-(OCH3)2 | 24.19 | 13.03 | 10.29 | NA | NA | 5.08 |
| B7 | 3,4,5-(OCH3)2 | 24.77 | 34.97 | 23.38 | 36.36 | 21.00 | 54.60 |
| B8 | 4-CH3 | 11.39 | NA | 26.14 | 43.21 | 32.95 | NA |
| C1 | — | 88.91 | 30.16 | 14.11 | 74.53 | 49.59 | NA |
| C2 | — | 79.49 | 26.57 | NA | 10.38 | 78.31 | NA |
| Compd. | R | Growth inhibition/% | |||||
|---|---|---|---|---|---|---|---|
| HCT116 | SW620 | BEL7402 | HepG2 | MCF-7 | A549 | ||
| He | — | 26.21 | 24.22 | 31.19 | 19.69 | 32.43 | NA |
| A1 | 4-H | 46.13 | 24.42 | 34.24 | 39.81 | NA | NA |
| A2 | 4-Cl | NA | 28.11 | 30.67 | NA | 17.38 | NA |
| A3 | 3,4-Cl2 | 31.23 | 20.86 | NA | 56.07 | NA | 32.54 |
| A4 | 4-OCH3 | NA | NA | NA | NA | 36.86 | NA |
| A5 | 4-NO2 | 90.48 | 27.22 | 39.52 | 53.79 | 11.00 | 44.60 |
| B1 | 4-H | 29.55 | NA | 17.24 | 32.26 | 37.48 | 25.71 |
| B2 | 4-F | 28.77 | NA | 53.10 | 34.31 | 34.81 | NA |
| B3 | 4-Cl | NA | 12.81 | 27.10 | 31.26 | 44.29 | 23.73 |
| B4 | 3,4-Cl2 | NA | 17.94 | NA | NA | 45.76 | 55.56 |
| B5 | 4-OCH3 | NA | 44.08 | NA | 46.43 | 48.24 | 41.75 |
| B6 | 3,4-(OCH3)2 | 24.19 | 13.03 | 10.29 | NA | NA | 5.08 |
| B7 | 3,4,5-(OCH3)2 | 24.77 | 34.97 | 23.38 | 36.36 | 21.00 | 54.60 |
| B8 | 4-CH3 | 11.39 | NA | 26.14 | 43.21 | 32.95 | NA |
| C1 | — | 88.91 | 30.16 | 14.11 | 74.53 | 49.59 | NA |
| C2 | — | 79.49 | 26.57 | NA | 10.38 | 78.31 | NA |
| Compd. | R | IC50 valueb/(μmol•L–1) | ||
|---|---|---|---|---|
| HCT116 | HepG2 | MCF-7 | ||
| A5 | 4-NO2 | 2.05 | 27.89 | >30 |
| C1 | — | 16.02 | 16.31 | >30 |
| C2 | — | 18.12 | >30 | 19.12 |
| 5-FU | — | 24.80 | 10.93 | 28.11 |
| Compd. | R | IC50 valueb/(μmol•L–1) | ||
|---|---|---|---|---|
| HCT116 | HepG2 | MCF-7 | ||
| A5 | 4-NO2 | 2.05 | 27.89 | >30 |
| C1 | — | 16.02 | 16.31 | >30 |
| C2 | — | 18.12 | >30 | 19.12 |
| 5-FU | — | 24.80 | 10.93 | 28.11 |
| Compd. | R | IC50a/(μmol•L–1) | SIb | ||
|---|---|---|---|---|---|
| HCT116 | HepG2 | MCF-7 | |||
| A5 | 4-NO2 | >30 | 14.63 | 1.07 | NT |
| C1 | — | >30 | 1.87 | 1.84 | NT |
| C2 | — | >30 | 1.65 | NT | 1.57 |
| 5-FU | 19.12 | 0.77 | 1.75 | 0.68 | |
| Compd. | R | IC50a/(μmol•L–1) | SIb | ||
|---|---|---|---|---|---|
| HCT116 | HepG2 | MCF-7 | |||
| A5 | 4-NO2 | >30 | 14.63 | 1.07 | NT |
| C1 | — | >30 | 1.87 | 1.84 | NT |
| C2 | — | >30 | 1.65 | NT | 1.57 |
| 5-FU | 19.12 | 0.77 | 1.75 | 0.68 | |
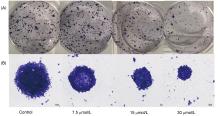

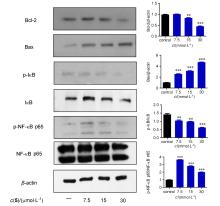



| [1] |
Bonsignore, G.; Patrone, M.; Grosso, F.; Martinotti, S.; Ranzato, E. Int. J. Mol. Sci. 2021, 22, 10380.
doi: 10.3390/ijms221910380 |
| [2] |
Sung, H.; Siegel, R. L.; Jemal, A.; Ferlay, J.; Laversanne, M.; Soerjomataram, I.; Bray, F. Ca-Cancer J. Clin. 2021, 71, 209.
doi: 10.3322/caac.21660 |
| [3] |
Boshuizen, J.; Peeper, D. S. Mol. Cell 2020, 78, 1002.
doi: S1097-2765(20)30351-8 pmid: 32559422 |
| [4] |
Choi, H.; Cho, S. Y.; Pak, H. J.; Kim, Y.; Choi, J. Y.; Lee, Y. J.; Gong, B. H.; Kang, Y. S.; Han, T.; Choi, G.; Cho, Y.; Lee, S.; Ryoo, D.; Park, H. J. Cheminf. 2017, 9, 2.
doi: 10.1186/s13321-016-0188-5 |
| [5] |
Sauter, E. R. Expert Rev. Clin. Pharmacol. 2020, 13, 265.
doi: 10.1080/17512433.2020.1738218 |
| [6] |
Wei, Y.; Ma, C. M.; Chen, D. Y.; Hattori, M. Phytochemistry (Elsevier) 2008, 69, 1875.
doi: 10.1016/j.phytochem.2008.03.004 |
| [7] |
Kang, H. R.; Eom, H. J.; Lee, S. R.; Choi, S. U.; Kang, K. S.; Lee, K. R.; Kim, K. H. Nat. Prod. Commun. 2015, 10, 1929.
pmid: 26749829 |
| [8] |
Rodriguez-Hernandez, D.; Demuner, A. J.; Barbosa, L. C. A.; Csuk, R.; Heller, L. Eur. J. Med. Chem. 2015, 105, 57.
doi: 10.1016/j.ejmech.2015.10.006 |
| [9] |
Zhang, R. H.; Jin, R.; Deng, H.; Shen, Q. K.; Quan, Z. S.; Jin, C. M. Korean J. Parasitol. 2021, 59, 297.
doi: 10.3347/kjp.2021.59.3.297 |
| [10] |
Bock, V. D.; Speijer, D.; Hiemstra, H.; Van Maarseveen, J. H. Org. Biomol. Chem. 2007, 5, 971.
doi: 10.1039/b616751a |
| [11] |
Liu, X. J.; Zhang, H. J.; Quan, Z. S. Med. Chem. Res. 2017, 26, 1935.
doi: 10.1007/s00044-017-1896-8 |
| [12] |
Zhang, G. R.; Ren, Y.; Yin, X. M.; Quan, Z. S. Lett. Drug Des. Discovery 2018, 15, 406.
doi: 10.2174/1570180814666170619094408 |
| [13] |
Shao, Y. P.; Han, R. B.; Wu, H. F.; Piao, F. Y. Med. Chem. Res. 2018, 27, 642.
doi: 10.1007/s00044-017-2089-1 |
| [14] |
Huang, X.; Chen, T.; Han, R. B.; Piao, F. Y. CNS Neurol. Disord.: Drug Targets 2018, 17, 448.
doi: 10.2174/1871527317666180704101332 |
| [15] |
Yan, G. F.; Ying, H. L.; Yang, L.; Fang, L. Y.; Xuan, Y. Y.; Jin, C. H.; Ji, Z. C.; Wang, M.; Ai, J.; Guan, P. M.; Piao, H. R.; Jin, C. M.; Jin, C. H. ChemMedChem 2021, 16, 2354.
doi: 10.1002/cmdc.202100122 pmid: 33738962 |
| [16] |
Zhang, T. Y.; Li, C. S.; Cui, M. Y.; Bai, X. Q.; Chen, J. H.; Song, Z. W.; Feng, B.; Liu, X. K. Mol. Diversity 2021, 25, 861.
doi: 10.1007/s11030-020-10071-9 |
| [17] |
Zhang, T. Y.; Li, C. S.; Li, P.; Bai, X. Q.; Guo, S. Y.; Jin, Y.; Piao, S. J. Mol. Diversity 2022, 26, 27.
doi: 10.1007/s11030-020-10154-7 |
| [18] |
Pang, L.; Liu, C. Y.; Gong, G. H.; Quan, Z. S. Acta Pharm. Sin. B 2020, 10, 628.
doi: 10.1016/j.apsb.2019.09.002 pmid: 32322467 |
| [19] |
Ma, Q. Q.; Bian, M.; Gong, G. H.; Bai, C. M.; Liu, C. Y.; Wei, C. X.; Quan, Z. S.; Du, H. H. J. Nat. Prod. 2022, 85, 15.
doi: 10.1021/acs.jnatprod.1c00377 |
| [20] |
Liu, H. J.; Huang, X.; Shen, Q. K.; Deng, H.; Li, Z.; Quan, Z. S. Iran. J. Pharm. Res. 2021, 20, 144.
|
| [21] |
Guo, H. Y.; Xing, Y.; Suna, Y. Q.; Liua, C.; Xua, Q.; Shang, F. F.; Zhang, R. H.; Jin, X. J.; Lee, F. C. J. J.; Kang, D.; Shen, Q. K.; Quan, Z. S. J. Ginseng Res. 2022.
|
| [22] |
Shang, F. F.; Wang, M. Y.; Ai, J. P.; Shen, Q. K.; Guo, H. Y.; Jin, C. M.; Chen, F. E.; Quan, Z. S.; Jin, L.; Zhang, C. Med. Chem. Res. 2021, 30, 2228.
doi: 10.1007/s00044-021-02803-9 |
| [23] |
Zhang, H. B.; Shen, Q. K.; Wang, H.; Jin, C. M.; Jin, C. M.; Quan, Z. S. Eur. J. Med. Chem. 2018, 158, 414.
doi: 10.1016/j.ejmech.2018.08.087 |
| [24] |
Sheng, C.; Che, X.; Wang, W.; Wang, S.; Cao, Y.; Miao, Z.; Yao, J.; Zhang, W. Eur. J. Med. Chem. 2011, 46, 5276.
doi: 10.1016/j.ejmech.2011.03.019 |
| [25] |
Wei, Z. Y.; Chi, K. Q.; Wang, K. S.; Wu, J.; Liu, L. P.; Piao, H. R. Bioorg. Med. Chem. Lett. 2018, 28, 1797.
doi: 10.1016/j.bmcl.2018.04.021 |
| [26] |
Chi, K. Q.; Wei, Z. Y.; Wang, K. S.; Wu, J.; Chen, W. Q.; Jin, X. J.; Piao, H. R. Bioorg. Chem. 2017, 75, 157.
doi: 10.1016/j.bioorg.2017.09.013 |
| [27] |
Rashid, S.; Dar, B. A.; Majeed, R.; Hamid, A.; Bhat, B. A. Eur. J. Med. Chem. 2013, 66, 238.
doi: 10.1016/j.ejmech.2013.05.029 |
| [28] |
Shen, Q. K.; Liu, C. F.; Zhang, H. J.; Tian, Y. S.; Quan, Z. S. Bioorg. Med. Chem. Lett. 2017, 27, 4871.
doi: 10.1016/j.bmcl.2017.09.040 |
| [29] |
Wang, S. B.; Liu, H.; Li, G. Y.; Li, J.; Li, X. J.; Lei, K.; Wei, L. C.; Quan, Z. S.; Wang, X. K.; Liu, R. M. Pharmacol. Rep. 2019, 71, 1244.
doi: 10.1016/j.pharep.2019.07.011 |
| [30] |
Ghosh, K.; Sarkar, A. R.; Chattopadhyay, A. P. Eur. J. Org. Chem. 2012, 2012, 1311.
doi: 10.1002/ejoc.201101240 |
| [31] |
Wei, Z. Y.; Cui, B. R.; Cui, X.; Wu, Y. L.; Fu, Y.; Liu, L. P.; Piao, H. R. Chem. Biol. Drug Des. 2017, 89, 47.
doi: 10.1111/cbdd.12828 |
| [32] |
Liu, Y. X.; Cui, Z. P.; Liu, B.; Cai, B. L.; Li, Y. H.; Wang, Q. M. J. Agric. Food Chem. 2010, 58, 2685.
doi: 10.1021/jf902541w |
| [33] |
Zhang, L. H.; Zhang, Z. H.; Li, M. Y.; Wei, Z. Y.; Jin, X. J.; Piao, H. R. Bioorg. Med. Chem. Lett. 2019, 29, 1440.
doi: 10.1016/j.bmcl.2019.04.028 |
| [34] |
Lei, K. F.; Wu, Z. M.; Huang, C. H. Biosens. Bioelectron. 2015, 74, 878.
doi: 10.1016/j.bios.2015.07.060 |
| [35] |
Liu, Y.; Li, J.; Xu, H.; Zhang, Y.; Liu, Y.; Liu, X. Int. J. Mol. Med. 2009, 24, 653.
|
| [36] |
Han, Z. X.; Wang, H. M.; Jiang, G.; Du, X. P.; Gao, X. Y.; Pei, D. S. Cancer Biother. Radiopharm. 2013, 28, 398.
|
| [37] |
Zhang, T.; Saghatelian, A. Biochim. Biophys. Acta, Mol. Cell Biol. Lipids 2013, 1831, 1542.
|
| [38] |
Hwang, S. G.; Park, J.; Park, J. Y.; Park, C. H.; Lee, K. H.; Cho, J. W.; Hwang, J. I.; Seong, J. Y. PLoS One 2012, 7, e44259.
doi: 10.1371/journal.pone.0044259 |
| [39] |
Yenmis, G.; Yaprak Sarac, E.; Besli, N.; Soydas, T.; Tastan, C.; Dilek Kancagi, D.; Yilanci, M.; Senol, K.; Karagulle, O. O.; Ekmekci, C. G.; Ovali, E.; Tuncdemir, M.; Ulutin, T.; Kanigur Sultuybek, G. Acta Histochem. 2021, 123, 151709.
doi: 10.1016/j.acthis.2021.151709 |
| [40] |
Zerbini, L. F.; Wang, Y.; Czibere, A.; Correa, R. G.; Cho, J. Y.; Ijiri, K.; Wei, W.; Joseph, M.; Gu, X.; Grall, F.; Goldring, M. B.; Zhou, J. R.; Libermann, T. A. Proc. Natl. Acad. Sci. U. S. A. 2004, 101, 13618.
pmid: 15353598 |
| [41] |
Denlinger, C. E.; Rundall, B. K.; Jones, D. R. Semin. Thorac. Cardiovasc. Surg. 2004, 16, 28.
pmid: 15366685 |
| [42] |
Wang, Z.; Li, M. Y.; Mi, C.; Wang, K. S.; Ma, J.; Jin, X. Int. J. Mol. Sci. 2017, 18, 1619.
doi: 10.3390/ijms18081619 |
| [43] |
Bian, M.; Zhen, D.; Shen, Q. K.; Du, H. H.; Ma, Q. Q.; Quan, Z. S. Bioorg. Chem. 2021, 107, 104598.
doi: 10.1016/j.bioorg.2020.104598 |
| [44] |
Ko, J. H.; Lee, J. H.; Jung, S. H.; Lee, S. G.; Chinnathambi, A.; Alharbi, S. A.; Yang, W. M.; Um, J. Y.; Sethi, G.; Ahn, K. S. Molecules 2017, 22, 1157.
doi: 10.3390/molecules22071157 |
| [1] | Ruixia Cao, Yuping Jia. Synthesis and Biological Activity of Novel Pyrrolo[2,3-d]pyrimidine Derivatives Containing Coumarin [J]. Chinese Journal of Organic Chemistry, 2023, 43(9): 3304-3311. |
| [2] | Weishu Zhang, Lifei Nie, Khurshed Bozorov, Haji Akber Aisa, Jiangyu Zhao. Synthesis of Diethyl 2,5-Diaminothiophene-3,4-dicarboxylate Derivatives and Antitumor Activity Study [J]. Chinese Journal of Organic Chemistry, 2023, 43(7): 2543-2552. |
| [3] | Jing Zhao, Zhe Jin, Run Wang, Xin'geng Zhang, Yingmei Han, Chun Hu, Xiaoping Liu, Chuanming Zhang, Liping Jin. Design, Synthesis and Anticancer Activity of 2-((Pyridin- 2-ylmethyl)thio)-1H-benzimidazole Derivatives [J]. Chinese Journal of Organic Chemistry, 2022, 42(7): 2172-2183. |
| [4] | Junzhe Yang, Zichao Xu, Chunpu Li, Yuanzheng Cheng, Hong Liu. Research Progress of Tropomyosin Receptor Kinase (TRK) Inhibitors [J]. Chinese Journal of Organic Chemistry, 2022, 42(7): 2055-2069. |
| [5] | Yan Zeng, Lifei Nie, Chao Niu, Aytilla Mamatjan, Khurshed Bozorov, Jiangyu Zhao, Haji Akber Aisa. Synthesis and Biological Activities of Dihydrooxazolo[5,4-d]-pyrrolo[1,2-a]pyrimidinones [J]. Chinese Journal of Organic Chemistry, 2022, 42(2): 543-556. |
| [6] | Yajun Mao, Xiangmin Shao, Yangjie Li, Ruimei Cao, Yali Feng, Guangyu Zhai. Research Progress on the Synthesis of Quercetin Derivatives [J]. Chinese Journal of Organic Chemistry, 2022, 42(11): 3588-3605. |
| [7] | Xin Liu, Runmei Xu, Lin Wang, Yaxue Liu, Zhihao Chen, Wei Qin, Yushun Tian. Synthesis and Evaluation in vitro of Dihydrothiophenopyridine-Chalcone Derivatives as Anticancer Activity Agents [J]. Chinese Journal of Organic Chemistry, 2021, 41(9): 3550-3559. |
| [8] | Shenghui Wang, Yongfeng Guan, Xiujuan Liu, Xinying Yuan, Guangxi Yu, Yinru Li, Yanbing Zhang, Jian Song, Wen Li, Saiyang Zhang. Design, Synthesis and Anticancer Activity Studies of Novel Quinoline-Indole Derivatives [J]. Chinese Journal of Organic Chemistry, 2021, 41(9): 3617-3624. |
| [9] | Maihesuti Liwaliding, Yu Junjie, Wang Qi, Cao Jianguo, Huang Guozheng. Synthesis and Anticancer Evaluation of Amido-Derivative of Hydnocarpin D [J]. Chinese Journal of Organic Chemistry, 2020, 40(9): 2764-2771. |
| [10] | Shi Lei, Li Ziqiu, Cui Xinxin, Zhu Ting, Pang Xiaojing, Li Longhui, Luo Defu, Liu Fangfang, Zhao Bingyu, Long Yue, Zhang Saiyang. Synthesis and Anticancer Activity of Novel Coumarin Derivatives [J]. Chinese Journal of Organic Chemistry, 2020, 40(6): 1598-1607. |
| [11] | Wu Bowen, Cui Xinxin, Zhu Ting, Wang Shenghui, Lu Chaofan, Wang Jinjie, Dang Hexiang, Zhang Saiyang, Ding Li'na, Jin Chengyun. Design, Synthesis and Anticancer Activity Studies of Novel Trimethoxyphenyl-quinoline Derivatives [J]. Chinese Journal of Organic Chemistry, 2020, 40(4): 978-987. |
| [12] | Sun Xiaoyang, Feng Siran, Dong Jinjiao, Feng Jiajia, Liu Zhenming, Song Yali, Qiao Xiaoqiang. Design, Synthesis and Biological Activity of Pyrazolo[3,4-d]pyrimidine Derivatives Containing Indole Moiety [J]. Chinese Journal of Organic Chemistry, 2020, 40(2): 391-397. |
| [13] | Sheng Qiwei, Zhao Wanqiu, Zeng Ming, Xie Zhongpao, Xia Yaping, Cui Dongmei. Synthesis and Evaluation of Chalcone Derivatives as Novel Anticancer Agents [J]. Chin. J. Org. Chem., 2019, 39(3): 703-708. |
| [14] | Mao Zewei, Liu Bei, Zhu Ping, Zhang Lijun, Zhu Jiahong, Wu Linze, Wan Chunping. Synthesis and Biological Evaluation of Piperazine Substituted 3-Aryl-5-furanyldihydropyrazole Amide Derivatives [J]. Chin. J. Org. Chem., 2018, 38(8): 2167-2173. |
| [15] | Huang Xinwei, Liu Jianli. Synthesis and Anticancer Activities of Novel Pyranocoumarin Fused Pyrimidine Based on Cyanoenamine [J]. Chin. J. Org. Chem., 2018, 38(5): 1233-1241. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||