Chinese Journal of Organic Chemistry ›› 2024, Vol. 44 ›› Issue (5): 1480-1493.DOI: 10.6023/cjoc202310008 Previous Articles Next Articles
REVIEWS
徐光利a,b,c,*( ), 韩鸿萍a,b, 曹露微a, 洪思敏a, 海林悦a, 崔香a
), 韩鸿萍a,b, 曹露微a, 洪思敏a, 海林悦a, 崔香a
收稿日期:2023-10-07
修回日期:2023-12-12
发布日期:2023-12-21
基金资助:
Guangli Xua,b,c( ), Hongping Hana,b, Luwei Caoa, Simin Honga, linyue Haia, Xiang Cuia
), Hongping Hana,b, Luwei Caoa, Simin Honga, linyue Haia, Xiang Cuia
Received:2023-10-07
Revised:2023-12-12
Published:2023-12-21
Contact:
*E-mail: Supported by:Share
Guangli Xu, Hongping Han, Luwei Cao, Simin Hong, linyue Hai, Xiang Cui. Research Progress of Transition Metal-Catalyzed Synthesis of 1,3-Conjugated Dienyl Boron Compounds[J]. Chinese Journal of Organic Chemistry, 2024, 44(5): 1480-1493.
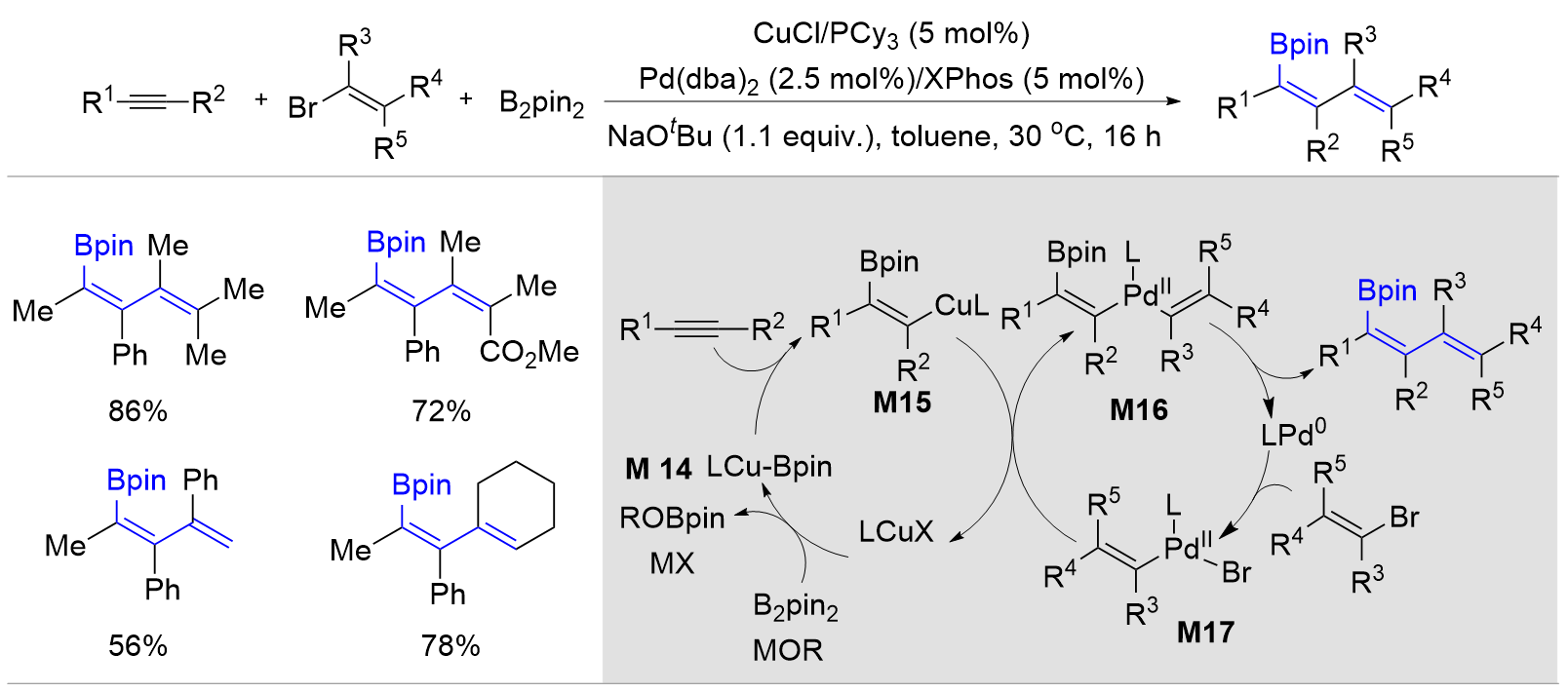
| [1] |
(a) Grigalunas, M.; Ankner, T.; Norrby, P.-O.; Wiest, O.; Helquist, P. Org. Lett. 2014, 16, 3970.
doi: 10.1021/ol5017965 pmid: 25032503 |
|
(b) Huang, E.; Bunel, E.; Faul, M. F. Org. Lett. 2007, 9, 4343.
pmid: 25032503 |
|
| [2] |
(a) Nicholas, C. P. Appl. Catal., A 2017, 543, 82.
|
|
(b) Kotha, S.; Meshram, M. Chem.-Asian J. 2018, 13, 1758.
|
|
| [3] |
(a) Sun, H.; Liang, Y.; Thompson, M. P.; Gianneschi, N. C. Prog. Polym. Sci. 2021, 120, 101427.
|
|
(b) Chen, M.; Yang, B.; Chen, C. Synlett 2016, 27, 1297.
|
|
|
(c) Ishii, S.; Ota, Y.; Matsuoka, S.; Suzuki, M. ACS Appl. Polym. Mater. 2023, 5, 7614.
|
|
| [4] |
Chen, J.-M.; Yu, B.; Wei, Y.-M. Appl. Energy 2018, 224, 160.
|
| [5] |
(a) Müller, K.; Faeh, C.; Diederich, F. Science 2007, 317, 1881.
doi: 10.1126/science.1131943 pmid: 26756377 |
|
(b) Purser, S.; Moore, P. R.; Swallow, S.; Gouverneur, V. Chem. Soc. Rev. 2008, 37, 320.
pmid: 26756377 |
|
|
(c) Gillis, E. P.; Eastman, K. J.; Hill, M. D.; Donnelly, D. J.; Meanwell, N. A. J. Med. Chem. 2015, 58, 8315.
doi: 10.1021/acs.jmedchem.5b00258 pmid: 26756377 |
|
|
(d) Wang, J.; Sánchez-Roselló, M.; Aceña, J. L.; Pozo, C.; rochinsky, A. E.; Fustero, S.; Soloshonok, V. A.; Liu, H. Chem. Rev. 2014, 114, 2432.
pmid: 26756377 |
|
|
(e) Zhou, Y.; Wang, J.; Gu, Z.; Wang, S.; Zhu, W.; Aceña, J. L.; loshonok, V. A.; Izawa, K.; Liu, H. Chem. Rev. 2016, 116, 422.
doi: 10.1021/acs.chemrev.5b00392 pmid: 26756377 |
|
|
(f) Hu, J.; Yang, Y.; Lou, Z.; Ni, C.; Hu, J. Chin. J. Chem. 2018, 36, 1202.
pmid: 26756377 |
|
| [6] |
(a) Hedhli, A.; Baklouti, A. Tetrahedron Lett. 1995, 36, 4433.
pmid: 8399151 |
|
(b) Sporn, M. B.; Dunlop, N. M.; Newton, D. L.; Henderson, W. R. Nature 1976, 263, 110.
pmid: 8399151 |
|
|
(c) Zhu, Y.; Liu, R. S. H. Biochemistry 1993, 32, 10233.
pmid: 8399151 |
|
|
(d) Chopra, D. P.; Wilkoff, W. J. Eur. J. Cancer 1979, 15, 1417.
pmid: 8399151 |
|
|
(e) Srisethnil, S. J. Med. Chem. 1979, 22, 1059.
pmid: 8399151 |
|
| [7] |
(a) Ohmura, T.; Masuda, K.; Takase, I.; Suginome, M. J. Am. Chem. Soc. 2009, 131, 16624.
|
|
(b) Abbas, S. Y.; Zhao, P.; Overman, L. E. Org. Lett. 2018, 20, 868.
|
|
| [8] |
(a) Pyziak, J.; Walkowiak, J.; Marciniec, B. Chem.-Eur. J. 2017, 23, 3502.
|
|
(b) Sardini, S. R.; Lambright, A. L.; Trammel, G. L.; Omer, H. M.; Liu, P.; Brown, M. K. J. Am. Chem. Soc. 2019, 141, 9391.
|
|
|
(c) Yang, C.; Gao, Y.; Bai, S.; Jiang, C.; Qi, X. J. Am. Chem. Soc. 2020, 142, 11506.
|
|
| [9] |
(a) Stikute, A.; Turks, M. Tetrahedron Lett. 2017, 58, 2727.
pmid: 32427489 |
|
(b) Mario Altendorfera M; Menche, D. Chem. Commun. 2012, 48, 8267.
pmid: 32427489 |
|
|
(c) Gan, Y.; Hu, H.; Liu, Y.-H. Org. Lett. 2020, 22, 4418.
doi: 10.1021/acs.orglett.0c01424 pmid: 32427489 |
|
| [10] |
(a) Reid, W. B.; Spillane, J. J.; Krause, S. B.; Watson, D. A. J. Am. Chem. Soc. 2016, 138, 17, 5539.
pmid: 28161943 |
|
(b) Lovinger, G. J.; Aparece, M. D.; Morken, J. P. J. Am. Chem. Soc. 2017, 139, 3153.
doi: 10.1021/jacs.6b12663 pmid: 28161943 |
|
| [11] |
Altendorfera, M.; Menche, D. Chem. Commun. 2012, 48, 8267.
|
| [12] |
(a) Jiang, B.; Liang, Q. J.; Han, Y. Org. Lett. 2018, 20, 3215.
doi: 10.1021/acs.orglett.8b01067 pmid: 26382149 |
|
(b) Kischkewitz, M.; Gerleve, C.; Studer, A. Org. Lett. 2018, 20, 3666.
doi: 10.1021/acs.orglett.8b01459 pmid: 26382149 |
|
|
(c) Tripathi, C. B.; Mukherjee, S. Org. Lett. 2015, 17, 4424.
doi: 10.1021/acs.orglett.5b02026 pmid: 26382149 |
|
|
(d) Dada, R.; Wei, Z.; Gui, R. Angew. Chem., Int. Ed. 2018, 57, 3981.
pmid: 26382149 |
|
| [13] |
(a) Yang, X. H.; Dong, V. M. J. Am. Chem. Soc. 2017, 139, 1774.
|
|
(b) Marcum, J. S.; Roberts, C. C.; Manan, R. S. J. Am. Chem. Soc. 2017, 139, 15580.
|
|
|
(c) Roberts, C. C.; Matías, D. M.; Goldfogel, M. J. J. Am. Chem. Soc. 2015, 137, 6488.
|
|
| [14] |
(a) Guillam, A.; Toupet, L.; Maddaluno, J. J. Org. Chem. 1998, 63, 5110.
pmid: 20863073 |
|
(b) Gulías, M.; Durán, J.; López, F. J. Am. Chem. Soc. 2007, 129, 11026.
pmid: 20863073 |
|
|
(c) Fujiwara, K.; Kurahashi, T.; Matsubara, S. Org. Lett. 2010, 12, 4548.
doi: 10.1021/ol101842y pmid: 20863073 |
|
|
(d) Liu, L.; Kim, H.; Xie, Y. J. Am. Chem. Soc. 2017, 139, 13656.
pmid: 20863073 |
|
| [15] |
(a) Barluenga, J.; Tomás, G. M.; Aznar, F. Adv. Synth. Catal. 2010, 352, 3235.
pmid: 29110364 |
|
(b) Zhang, X. M.; Yang, J.; Zhuang, Q. B. ACS Catal. 2018, 8, 6094.
pmid: 29110364 |
|
|
(c) Kreyenschmidt, F.; Koszinowsk, K. Chem.-Eur. J. 2018, 24, 1168.
doi: 10.1002/chem.201704547 pmid: 29110364 |
|
| [16] |
(a) Lopez, S. J. A.; Lamberti, M.; Pappalardo, D. Macromol 2003, 36, 9260.
pmid: 23202137 |
|
(b) Milione, S.; Cuomo, C.; Capacchione, C. Macromol 2007, 40, 5638.
pmid: 23202137 |
|
|
(c) Luo, K.; Kim, S. J.; Cartwright, A. N. Macromol 2011, 44, 4665.
pmid: 23202137 |
|
|
(d) Bonnet, F.; Jones, C. E.; Semlali, C. Dalton Trans. 2013, 42, 790.
doi: 10.1039/c2dt31624b pmid: 23202137 |
|
|
(e) Kostjuk, S. V. RSC Adv. 2015, 5, 13125.
pmid: 23202137 |
|
| [17] |
Eberlin, L.; Tripoteau, F.; Carreaux, F.; Whiting, A.; Carboni, B. J. Org. Chem. 2014, 10, 237.
|
| [18] |
Wang, C.; Tobrman, T.; Xu, Z.; Negishi, E. Org. Lett. 2009, 18, 4092.
|
| [19] |
Vargo, T. R.; Hale, J. S.; Nelson, S. G. Angew. Chem., Int. Ed. 2010, 49, 8678.
|
| [20] |
(a) Wang, Y.; Wu, Y.; Li, Y.; Tang, Y. Chem. Sci. 2017, 8, 3852.
|
|
(b) Sakakibara, R.; Itoh, K.; Fujii, H. J. Org. Chem. 2019, 84, 18, 11474.
|
|
| [21] |
(a) Manolikakes, G.; Knochel, P. Angew. Chem., Int. Ed., 2008, 48, 205.
|
|
(b) Arthurs, R. A.; Hughes, D. L.; Richards, C. J. Eur. J. Inorg. Chem. 2022, 2022, e202101077.
|
|
| [22] |
(a) Liu, Z.; Luan, N.; Shen, L.; Li, J.; Zou, D.; Wu, Y.; Wu, Y. J. Org. Chem. 2019, 84, 12358.
|
|
(b) Goebel, J. F.; Zeng, Z.; Handelmann, J.; Hermann, A.; Rodstein, I.; Gensch, T.; Gessner, V. H.; Löffler, L. Angew. Chem., Int. Ed. 2023, 62, e202216160
|
|
| [23] |
(a) Chishiro, A.; Konishi, M.; Inaba, R.; Yumura, T.; Imoto, H.; Naka, K. Dalton Trans. 2022, 51, 95.
|
|
(b) Chang, Y.-T.; Liu, L.-J.; Peng, W.-S.; Lin, L.-T.; Chan, Y.-T.; Tsai, F.-Y. J. Chin. Chem. Soc. 2021, 68, 469.
|
|
| [24] |
(a) Wang, X.; Sun, H.; Liu, J.; Zhong, W.; Zhang, M.; Zhou, H.; Dai, D.; Lu, X. Org. Lett. 2019, 21, 719.
|
|
(b) Markham, T. E.; Duggan, P. J.; Johnston, M. R. Tetrahedron 2023, 130, 133160
|
|
| [25] |
Stewart, S. K.; Whiting, A. Tetrahedron Lett. 1995, 36, 3925.
|
| [26] |
Coleman, R. S.; Walczak, M. C. Org. Lett. 2005, 7, 2289.
pmid: 15901191 |
| [27] |
(a) Maikhuri, V. K.; Maity, J.; Srivastavac, S.; Prasad, A. K. Org. Biomol. Chem. 2022, 20, 9522.
doi: 10.1039/d2ob01646j pmid: 36412483 |
|
(b) Dang, H. T.; Nguyen, V. D.; Pham, H. H.; Arman, H. D.; Larionov, O. V. Tetrahedron 2019, 75, 3258
pmid: 36412483 |
|
| [28] |
Daini, M.; Suginome, M. Chem. Commun. 2008, 5224.
|
| [29] |
Wang, C.; Tobrman, T.; Xu, Z.; Negishi, E. Org. Lett. 2009, 11, 4092.
|
| [30] |
Negishi, E.; Tobrman, T.; Rao, H.; Xu, S.; Lee, C.-T. Isr. J. Chem. 2010, 50, 696.
|
| [31] |
Wang, C.; Xu, Z.; Tobrman, T.; Negishi, E. Adv. Synth. Catal. 2010, 352, 627.
|
| [32] |
Singidi, R. R.; RajanBabu, T. V. Org. Lett. 2010, 12, 2622.
|
| [33] |
Xu, S.; Zhang, Y.; Li, B.; Liu, S.-Y. J. Am. Chem. Soc. 2016, 138, 14566.
|
| [34] |
Wang, Z.; Wu, J.; Lamine, W.; Li, B., Sotiropoulos, J.-M.; Chrostowska, A.; Miqueu, K.; Liu, S.-Y. Angew. Chem., Int. Ed. 2021, 60, 21231.
|
| [35] |
Wang, Z.; Lamine, W. M.; Liu, S.-Y. Chem. Sci. 2023, 14, 2082.
|
| [36] |
Sasaki, Y.; Horita, Y.; Zhong, C.; Sawamura, M.; Ito, H. Angew. Chem., Int. Ed. 2011, 50, 2778.
|
| [37] |
Xu, H.-D.; Wu, H.; Jiang, C.; Chen, P.; Shen, M.-H. Tetrahedron Lett. 2016, 57, 2915.
|
| [38] |
Tai, C.-C.; Yu, M.-S.; Chen, Y.-L.; Chuang, W.-H.; Lin, T.-H. Yap, G. P. A.; Ong, T.-G. Chem. Commun. 2014, 50, 4344.
|
| [39] |
Mohan, B.; Park, K. H. Appl. Catal.,A 2016, 519, 78.
|
| [40] |
Moncomble, A.; Floch, P. L.; Lledos, A.; Gosmini, C. J. Org. Chem. 2012, 77, 5056.
doi: 10.1021/jo3005149 pmid: 22591028 |
| [41] |
Bassler, D. P.; Alwali, A.; Spence, L.; Beale, O.; Beng, T. K. J. Organomet. Chem. 2015, 780, 6.
|
| [42] |
Huang, Q.; Hu, M.-Y.; Zhu, S.-F. Org. Lett. 2019, 21, 7883.
|
| [43] |
Alfaro, R.; Parra, A.; Aleman, J.; Ruano, J. L. G.; Tortosa, M. J. Am. Chem. Soc. 2012, 134, 15165.
|
| [44] |
Rivera-Chao, E.; Mastral, M. Angew. Chem., Int. Ed. 2018, 57, 9945.
doi: 10.1002/anie.201806334 pmid: 29905396 |
| [45] |
Galiñanes, V.-N.; Mastral, M. F. ChemCatChem 2018, 10, 4817.
|
| [46] |
Xu, W.-Y.; Li, Y.-J.; Gong, T.-J.; Fu, Y. Org. Lett. 2022, 24, 5884.
|
| [47] |
Walkowiak, J.; Jankowska-Wajda, M.; Marciniec, B. Chem.- Eur. J. 2008, 14, 6679.
|
| [48] |
Pyziak, J.; Walkowiak, J.; Hoffmann, M.; Marciniec, B. J. Organomet. Chem. 2015, 794, 96e103
|
| [49] |
Hirano, M.; Kuramochi, A.; Shimada, K.; Komine, N.; Kiyota, S.; Westcott, S. A. Chem. Commun. 2019, 55, 10527.
|
| [50] |
Rohde, L. N.; Diver, S. T. J. Org. Chem. 2022, 87, 14078.
|
| [51] |
Kirai, N.; Iguchi, S.; Ito, T.; Takaya, J.; Iwasawa, N. Bull. Chem. Soc. Jpn. 2013, 86, 784.
|
| [52] |
Funk, T. W.; Efskind, J.; Grubbs, R. H. Org. Lett. 2005, 7, 187.
|
| [53] |
Zhang, L.; Yan, J.; Ahmadli, D.; Wang, Z.; Ritter, T. J. Am. Chem. Soc. 2023, 145, 20182.
doi: 10.1021/jacs.3c07119 pmid: 37695320 |
| [54] |
Jia, J.; Yuan, F.; Zhang, Z.; Song, X.; Hu, F.; Xia, Y. Org. Lett. 2022, 24, 1985.
|
| [55] |
Gerdin, M.; Moberg, C. Org. Lett. 2006, 8, 2929.
|
| [56] |
Pang, X.; Shu, X.-Z. Chin. J. Chem. 2023, 41, 1637
|
| [57] |
Xu, G.-L.; Liu, C.-Y.; Pang, X.; Liu, X.-Y.; Shu, X.-Z. CCS Chem. 2022, 4, 864.
|
| [1] | Siyi Mi, Longlong Ma, Jianguo Liu. Research Progress of Continuous Flow Selective Hydrogenation Technology [J]. Chinese Journal of Organic Chemistry, 2024, 44(5): 1445-1457. |
| [2] | Xiaolin Jiang, Chaoyang Wang, Liyuan Wu, Yuehui Li. Advances in Catalytic Conversion of CO2 with Carbazole-Based Molecules and Polymers [J]. Chinese Journal of Organic Chemistry, 2024, 44(5): 1423-1444. |
| [3] | Yuxuan Yan, Wanqing Lu, Huijun Qian, Leiyang Lv, Zhiping Li. Pd-Catalyzed Ring-Opening of gem-Difluorocyclopropanes for the Mono- and Bis-fluoroallylation of 1,3-Dicarbonyls [J]. Chinese Journal of Organic Chemistry, 2024, 44(5): 1630-1640. |
| [4] | Donghong Luo, Ping Li, Zhicai Chen, Jiayi Yang, Mengfan Sun, Juyou Lu. Pd-Catalyzed Cross-Coupling of o-Carboranyl Pyridyl Halides: Synthesis of o-Carboranyl Biaryls, Aminopyridines and Alkynylpyridine Derivatives [J]. Chinese Journal of Organic Chemistry, 2024, 44(5): 1568-1575. |
| [5] | Chenguang Liu. Progress in Asymmetric Hydrogenation of Aromatic N-Heterocyclic Compounds [J]. Chinese Journal of Organic Chemistry, 2024, 44(5): 1403-1422. |
| [6] | Chonglei Ji, Dewei Gao. Recent Advances in Catalytic Asymmetric Synthesis of Chiral 1,2-Bis(boronic) Esters [J]. Chinese Journal of Organic Chemistry, 2024, 44(5): 1385-1402. |
| [7] | Yongwei Cui, Chunmiao Liang, Haitao Zhu, Chengping Shen, Feiyang Ren, Menghan Sun, Yuan Zhao, Wenjing Wang, Dongmei Wang, Nini Zhou. cis-Selective [5+2]-Cycloaddition Reactions of Cyclic Morita-Baylis- Hillman Alcohols and Its Analogues with Arylethylenes Catalyzed by Ag(I) [J]. Chinese Journal of Organic Chemistry, 2024, 44(5): 1535-1548. |
| [8] | Xiangxue Cao, Yahui Jia, Shiji Yin, Liang Xu, Yu Wei, Huanhuan Song. Visible-Light-Induced C—C Bond Cleavage of Dihydroquinazolinones with Trifluoromethyl-Substituted Olefins Defluorinated Alkylation Reactions [J]. Chinese Journal of Organic Chemistry, 2024, 44(5): 1549-1557. |
| [9] | Duyi Shen, Linghui Li, Ge Jing, Yujia Liang, Xinhui Zhang, Peiwei Gong, Fanjun Zhang, Mianran Chao. Advances in Flavin-Inspired Photocatalytic Oxidations Involving Single Electron Transfer Process [J]. Chinese Journal of Organic Chemistry, 2024, 44(4): 1069-1093. |
| [10] | Tianfeng Peng, Yuxiang Zhao, Shaojian Pu, Juan Luo, Teng Liu, Yingchun Miao, Xianfu Shen. Recent Advances in Total Synthesis of Prenylated Indole Alkaloids by Transition Metal-Catalyzed Reactions as the Key Step [J]. Chinese Journal of Organic Chemistry, 2024, 44(4): 1160-1180. |
| [11] | Junjun Liu, Taotao Lu, Ping Ma, Qingyang Zhao, Fuk Yee Kwong. Palladium-Catalyzed C(sp3)—Si Bonds Transformation for Construct-ing Trifluoropropyl (Hetero)arenes through C(sp3)—C(sp2) Cross-Coupling Reactions [J]. Chinese Journal of Organic Chemistry, 2024, 44(4): 1319-1326. |
| [12] | Yunhui Wan, Fumei Yang, Minghan Chen, Deli Sun, Danfeng Ye. Esterification of N-Benzyl-N-t-butoxycarbonyl-amides and Unsaturated Alcohol under Transition Metal-Free Conditions [J]. Chinese Journal of Organic Chemistry, 2024, 44(4): 1293-1300. |
| [13] | Chen-Long Li, Zhi-Xiang Yu. Progress in Transition-Metal-Catalyzed Carbonylative Cycloadditions Using Carbon Monoxide [J]. Chinese Journal of Organic Chemistry, 2024, 44(4): 1045-1068. |
| [14] | Kaijie Guo, Xinshu Fu, Jing Li, Yan Chen, Meili Hu, Xihua Du, Yuyang Xie, Yan He. Recent Advances in Transition-Metal-Catalyzed C—S Bond Activation and Transformations [J]. Chinese Journal of Organic Chemistry, 2024, 44(4): 1124-1150. |
| [15] | Mengying Hou, Ai'e Wang, Peiqiang Huang. Recent Progress in Homogeneous Catalytic Hydrogenation of Nitro Compounds [J]. Chinese Journal of Organic Chemistry, 2024, 44(4): 1094-1105. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||