Chinese Journal of Organic Chemistry ›› 2025, Vol. 45 ›› Issue (4): 1315-1324.DOI: 10.6023/cjoc202407044 Previous Articles Next Articles
ARTICLES
冬海洋, 蒲佳欣, 李珂珂, 张昊文, 耿晓晴, 梁停停*( ), 王建红*(
), 王建红*( )
)
收稿日期:2024-07-29
修回日期:2024-09-14
发布日期:2024-10-29
基金资助:
Haiyang Dong, Jiaxin Pu, Keke Li, Haowen Zhang, Xiaoqing Geng, Tingting Liang( ), Jianhong Wang(
), Jianhong Wang( )
)
Received:2024-07-29
Revised:2024-09-14
Published:2024-10-29
Contact:
* E-mail: Supported by:Share
Haiyang Dong, Jiaxin Pu, Keke Li, Haowen Zhang, Xiaoqing Geng, Tingting Liang, Jianhong Wang. Synthesis and Bioactivity Study of Benzimidazole Derivatives with Fluorescent Self-Labeling and Tubulin Polymerization Inhibition[J]. Chinese Journal of Organic Chemistry, 2025, 45(4): 1315-1324.
| Compd. | R1 | R2 | Inhibition rate/% | |
|---|---|---|---|---|
| 5 µmol/L | 10 µmol/L | |||
| IV-01 | 3,4,5-(OMe)3 | 2-OH-4-OMe | 40.08 | 65.38 |
| IV-02 | 3,4,5-(OMe)3 | 2-OH-3-OMe | 70.81 | 72.70 |
| IV-03 | 3,4,5-(OMe)3 | 2-OH-5-OMe | 37.73 | 37.56 |
| IV-04 | 3,4,5-(OMe)3 | 2-OH | 31.87 | 34.03 |
| IV-05 | 3,4,5-(OMe)3 | H | 36.53 | 41.95 |
| IV-06 | 3,4,5-(OMe)3 | 4-OMe | 34.37 | 33.79 |
| IV-07 | 4-OMe | 2-OH-5-OMe | 38.05 | 34.42 |
| IV-08 | 4-OMe | 2-OH-4-OMe | 36.58 | 38.45 |
| IV-09 | 4-OMe | 2-OH-3-OMe | 29.22 | 25.67 |
| IV-10 | 4-OMe | 2-OH | 34.96 | 34.79 |
| IV-11 | 4-OMe | H | 41.04 | 32.92 |
| IV-12 | 4-OMe | 4-OMe | 37.26 | 38.38 |
| IV-13 | 3,4-(OMe)2 | 2-OH-4-OMe | 37.91 | 33.03 |
| IV-14 | 3,4-(OMe)2 | 2-OH-5-OMe | 31.47 | 30.96 |
| IV-15 | 3,4-(OMe)2 | 2-OH | 30.01 | 33.86 |
| IV-16 | 3,4-(OMe)2 | 4-OMe | 34.85 | 32.03 |
| IV-17 | 3,4-(OMe)2 | H | 39.02 | 40.49 |
| Paclitaxelb | — | — | — | 1.43 |
| Compd. | R1 | R2 | Inhibition rate/% | |
|---|---|---|---|---|
| 5 µmol/L | 10 µmol/L | |||
| IV-01 | 3,4,5-(OMe)3 | 2-OH-4-OMe | 40.08 | 65.38 |
| IV-02 | 3,4,5-(OMe)3 | 2-OH-3-OMe | 70.81 | 72.70 |
| IV-03 | 3,4,5-(OMe)3 | 2-OH-5-OMe | 37.73 | 37.56 |
| IV-04 | 3,4,5-(OMe)3 | 2-OH | 31.87 | 34.03 |
| IV-05 | 3,4,5-(OMe)3 | H | 36.53 | 41.95 |
| IV-06 | 3,4,5-(OMe)3 | 4-OMe | 34.37 | 33.79 |
| IV-07 | 4-OMe | 2-OH-5-OMe | 38.05 | 34.42 |
| IV-08 | 4-OMe | 2-OH-4-OMe | 36.58 | 38.45 |
| IV-09 | 4-OMe | 2-OH-3-OMe | 29.22 | 25.67 |
| IV-10 | 4-OMe | 2-OH | 34.96 | 34.79 |
| IV-11 | 4-OMe | H | 41.04 | 32.92 |
| IV-12 | 4-OMe | 4-OMe | 37.26 | 38.38 |
| IV-13 | 3,4-(OMe)2 | 2-OH-4-OMe | 37.91 | 33.03 |
| IV-14 | 3,4-(OMe)2 | 2-OH-5-OMe | 31.47 | 30.96 |
| IV-15 | 3,4-(OMe)2 | 2-OH | 30.01 | 33.86 |
| IV-16 | 3,4-(OMe)2 | 4-OMe | 34.85 | 32.03 |
| IV-17 | 3,4-(OMe)2 | H | 39.02 | 40.49 |
| Paclitaxelb | — | — | — | 1.43 |
| Compd. | IC50b/(µmol•L–1) | ||||
|---|---|---|---|---|---|
| HT-29 | HT-1080 | HepG2 | A549 | A549/Taxol | |
| IV-02 | 8.56±4.14 | 2.34±1.01 | 1.10±0.30 | 0.18±0.08 | 0.39±0.04 |
| Compd. | IC50b/(µmol•L–1) | ||||
|---|---|---|---|---|---|
| HT-29 | HT-1080 | HepG2 | A549 | A549/Taxol | |
| IV-02 | 8.56±4.14 | 2.34±1.01 | 1.10±0.30 | 0.18±0.08 | 0.39±0.04 |



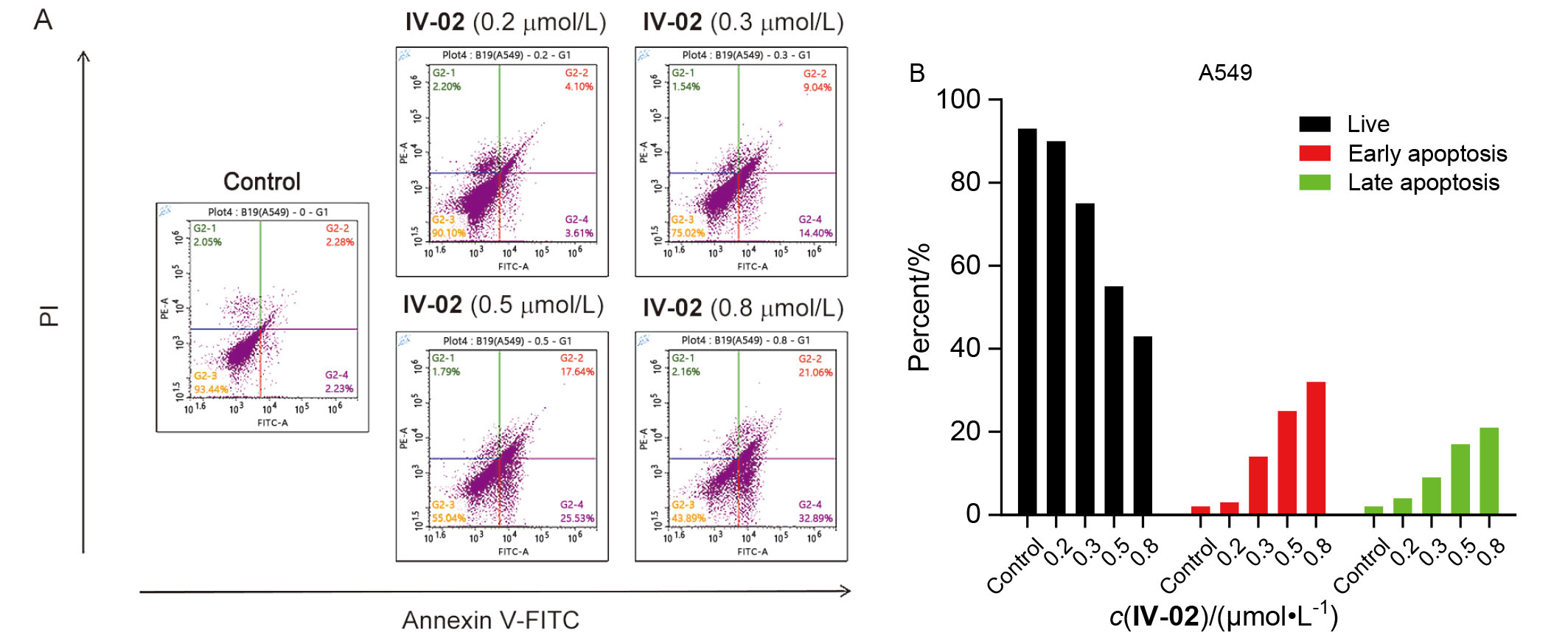


| [1] |
Hamel, E.; Lin, C. M. Biochem. Pharmacol. 1983, 32, 3864.
pmid: 6661259 |
| [2] |
Tron, G. C.; Pirali, T.; Sorba, G.; Pagliai, F.; Busacca, S.; Genazzani, A. A. J. Med. Chem. 2006, 49, 3033.
|
| [3] |
Cui, Y. J.; Liu, C.; Ma, C. C.; Ji, Y. T.; Yao, Y. L.; Tang, L. Q.; Zhang, C. M.; Wu, J. D.; Liu, Z. P. J. Med. Chem. 2020, 63, 14840.
|
| [4] |
Seddigi, Z. S.; Malik, M. S.; Saraswati, A. P.; Ahmed, S. A.; Babalghith, A. O.; Lamfon, H. A.; Kamal, A. MedChemComm 2017, 8, 1592.
doi: 10.1039/c7md00227k pmid: 30108870 |
| [5] |
Gaspari, R.; Prota, A. E.; Bargsten, K.; Cavalli, A.; Steinmetz, M. O. Chem 2017, 2, 102.
|
| [6] |
Pecnard, S.; Hamze, A.; Bignon, J.; Prost, B.; Deroussent, A.; Gallego-Yerga, L.; Peláez, R.; Paik, J. Y.; Diederich, M.; Alami, M.; Provot, O. Eur. J. Med. Chem. 2021, 223, 113656.
|
| [7] |
Bukhari, S. N. A.; Kumar, G. B.; Revankar, H. M.; Qin, H. L. Bioorg. Chem. 2017, 72, 130.
doi: S0045-2068(17)30128-1 pmid: 28460355 |
| [8] |
Shan, Y.; Zhang, J.; Liu, Z.; Wang, M.; Dong, Y. Curr. Med. Chem. 2011, 18, 523.
pmid: 21143124 |
| [9] |
Gu, Y. F.; Wu, C. J.; Wang, S. Q.; Zhang, S. L.; Lu, Y.; Si, X. X.; Jiang, B. L. Chin. J. Org. Chem. 2024, 44, 3497. (in Chinese)
|
|
(顾一飞, 吴彩菊, 王思琪, 张世琳, 陆远, 司鑫鑫, 蒋佰玲, 有机化学, 2024, 44, 3497.)
doi: 10.6023/cjoc202403049 |
|
| [10] |
Kremmidiotis, G.; Leske, A. F.; Lavranos, T. C.; Beaumont, D. Mol. Cancer Ther. 2010, 9, 1562.
doi: 10.1158/1535-7163.MCT-09-0815 pmid: 20515948 |
| [11] |
Flynn, B. L.; Gill, G. S.; Grobelny, D. W.; Chaplin, J. H.; Paul, D.; Leske, A. F.; Lavranos, T. C.; Chalmers, D. K.; Charman, S. A.; Kostewicz, E. J. Med. Chem. 2011, 54, 6014.
|
| [12] |
Wang, Q. H.; Arnst, K. E.; Wang, Y. X.; Kumar, G.; Ma, D. J.; White, S. W.; Miller, D. D.; Li, W. M.; Li, W. J. Med. Chem. 2019, 62, 6734.
|
| [13] |
Kashyap, V. K.; Wang, Q. H.; Setua, S.; Nagesh, P. K. B.; Chauhan, N.; Kumari, S.; Chowdhury, P.; Miller, D. D.; Yallapu, M. M.; Li, W.; Jaggi, M.; Hafeez, B. B.; Chauhan, S. C. J. Exp. Clin. Cancer Res. 2019, 38, 29.
doi: 10.1186/s13046-018-1009-7 pmid: 30674344 |
| [14] |
Lai, Q. H.; Wang, Y. X.; Wang, R. X.; Lai, W. R.; Tang, L. Z.; Tao, Y. R.; Liu, Y.; Zhang, R. R.; Huang, L. Y.; Xiang, H. T.; Zeng, S. X.; Gou, L. T.; Chen, H.; Yao, Y. Q.; Yang, J. L. Eur. J. Med. Chem. 2018, 156, 162.
|
| [15] |
Huo, Z.; Liu, K.; Zhang, X.; Liang, Y.; Sun, X. Eur. J. Med. Chem. 2022, 235, 114271.
|
| [16] |
Li, G.; Wu, J. Q.; Cai, X. J.; Guan, W.; Zeng, Z. J.; Ou, Y. H.; Wu, X. Y.; Li, J. Y.; Fang, X. X.; Liu, J. L.; Zhang, Y. L.; Wang, H. M.; Yin, C. Q.; Yao, H. L. Eur. J. Med. Chem. 2023, 252, 115284.
|
| [17] |
Karetnikov, G. L.; Vasilyeva, L. A.; Babayeva, G.; Pokrovsky, V. S.; Skvortsov, D. A.; Bondarenko, O. B. ACS Pharmacol. Transl. Sci. 2024, 7, 384.
doi: 10.1021/acsptsci.3c00239 pmid: 38357282 |
| [18] |
Tan, Y. C.; Hu, H.; Zhu, W. J.; Wang, T.; Gao, T.; Wang, H. Q.; Chen, J.; Xu, J. Y.; Xu, S. T.; Zhu, H. J. Eur. J. Med. Chem. 2023, 262, 115881.
|
| [19] |
Li, Y.; Ma, H. Q.; Wang, Y. L. Chin. J. Org. Chem. 2008, 28, 210. (in Chinese)
|
|
(李焱, 马会强, 王玉炉, 有机化学, 2008, 28, 210.)
|
|
| [20] |
Santosh, P. C.; Pandeya, S. N.; Pathak, A. K. Int. J. Res. Ayurveda Pharm. 2001, 2, 1726.
|
| [21] |
Dong, H. Y. Ph.D. Dissertation, Henan University, Kaifeng, 2024. (in Chinese)
|
|
(冬海洋. 博士论文, 河南大学, 开封, 2024).
|
| [1] | Mengjia Xiao, Xike Gao. Design, Synthesis and Anti-inflammatory Activity of Azulene Derivatives Containing Benzimidazole Unit [J]. Chinese Journal of Organic Chemistry, 2023, 43(9): 3246-3256. |
| [2] | Binghan Lin, Jibin Zhuo, Caixia Lin, Yong Gao, Yaofeng Yuan. Synthesis and Nucleotide Recognition Properties of Carborane-Based Benzoimidazolium Cyclophane [J]. Chinese Journal of Organic Chemistry, 2022, 42(8): 2551-2558. |
| [3] | Jing Zhao, Zhe Jin, Run Wang, Xin'geng Zhang, Yingmei Han, Chun Hu, Xiaoping Liu, Chuanming Zhang, Liping Jin. Design, Synthesis and Anticancer Activity of 2-((Pyridin- 2-ylmethyl)thio)-1H-benzimidazole Derivatives [J]. Chinese Journal of Organic Chemistry, 2022, 42(7): 2172-2183. |
| [4] | Siyu Zhu, Xinyu Huo, Qin Ma, Wei Chen, Jie Zhang, Liang Guo. Design, Synthesis, and Antitumor Activity of β-Carboline-Benzimidazole Hybrids [J]. Chinese Journal of Organic Chemistry, 2022, 42(4): 1129-1135. |
| [5] | Xiaojing Tian, Zhenzhen Fan, Si Jiang, Zhiwei Li, Jiangsheng Li, Yuefei Zhang, Cuihong Lu, Weidong Liu. Metal-Free Synthesis of Benzimidazo[1,2-c]quinazolines from N-Cyanobenzimidazoles via Double C—H Functionalizations [J]. Chinese Journal of Organic Chemistry, 2022, 42(11): 3684-3692. |
| [6] | Yuyu Guo, Xiangjie Chen, Shiwu Li, Zhihua Cai, Lin He. Synthesis of Mutisubstituted Dihydropyridino[1,2-a]benzimidazole Derivatives via Tandem Reaction of 2-Arylbenzimidazoles [J]. Chinese Journal of Organic Chemistry, 2021, 41(9): 3692-3700. |
| [7] | Fengxing Li, Xin Lu, Xu Liu, Lulu Su, Xiaoliu Li, Hua Chen. Structural Modification of Benzimidazole-Iminosugars and Their Inhibitory Activities against β-Glycosidases [J]. Chinese Journal of Organic Chemistry, 2021, 41(9): 3643-3651. |
| [8] | Xu Liu, Lulu Su, Zhaoxi Zhou, Liping Niu, Ligang Gao, Huanhuan Ju, Fengxing Li, Xiaoliu Li, Hua Chen. Design and Synthesis of Benzimidazole-Iminosugars and Their Inhibitory Activities against Glycosidases [J]. Chinese Journal of Organic Chemistry, 2021, 41(7): 2861-2874. |
| [9] | Lin Mei, Wu Fan, Liu Tianhui, Chen Zhitao, Xu Xiuzhi, Ke Fang. Visible-Light Promoted Preparation of Benzimidazoles by Eosin Y Catalyzed Reaction of Benzonitrile Derivatives in Water [J]. Chinese Journal of Organic Chemistry, 2020, 40(8): 2563-2569. |
| [10] | Wang Xin, Li Guofeng, Sun Kai, Zhang Bing. Peroxide-Induced Radical Relay Carbocyclization towards Polycyclic Benzimidazole[2,1-a]isoquinolines [J]. Chinese Journal of Organic Chemistry, 2020, 40(4): 913-921. |
| [11] | Shen Jingru, Li Lihong, Wang Xiaogang, Zhang Jia'nan, Jiang Zhenglin. Green Synthesis of 2,2'-Bibenzazole and Its Polymers by Using Hexachloroacetone as C2 Synthon [J]. Chinese Journal of Organic Chemistry, 2020, 40(12): 4322-4327. |
| [12] | Yang Kai, Yao Chen, Gao Juanjuan, Chen Sihong, Zheng Xuejie, Deng Luxuan, Zhang Yu'na, Liu Meijuan, Wang Zhaoyang. Progress on the Synthesis of Pyrido[1,2-a]benzimidazoles [J]. Chinese Journal of Organic Chemistry, 2020, 40(12): 4168-4183. |
| [13] | Li Yingjun, Zhao Yue, Jin Kun, Gao Lixin, Sheng Li, Liu Xuejie, Yang Hongjing, Lin Ledi, Li Jia. Synthesis and PTP1B/TCPTP Inhibitory Activity Evaluation of Novel 2,5-Disubstituted-1,3,4-thiadiazolamide Derivatives Containing Carbazole/Benzimidazole Moity [J]. Chin. J. Org. Chem., 2019, 39(9): 2599-2608. |
| [14] | Li Yingjun, Zhao Yue, Jin Kun, Liu Jihong, Zhou Xiaoxia, Yang Kaidong. A Novel Benzimidazole-Hydrazone Derivative for Colorimetric and Fluorescent Recognition of F- and AcO- [J]. Chin. J. Org. Chem., 2019, 39(4): 1013-1022. |
| [15] | Yang Kang, Gu Huiwen, Zhang Fang, Xu Xiaojuan, Zhang Lijie, Sun Yaquan. Synthesis of 1,2,6-Trisubstituted Benzimidazoles [J]. Chin. J. Org. Chem., 2018, 38(8): 2130-2136. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||