Chinese Journal of Organic Chemistry ›› 2025, Vol. 45 ›› Issue (9): 3441-3449.DOI: 10.6023/cjoc202501013 Previous Articles Next Articles
ARTICLES
祝辉a, 吴鹏a, 钟晨鸣a, 李舒铭a, 林钢a, 刘雪粉b, 罗书平a,*( )
)
收稿日期:2025-01-15
修回日期:2025-04-11
发布日期:2025-05-15
基金资助:
Hui Zhua, Peng Wua, Chenming Zhonga, Shuming Lia, Gang Lina, Xuefen Liub, Shuping Luoa,*( )
)
Received:2025-01-15
Revised:2025-04-11
Published:2025-05-15
Contact:
E-mail: Supported by:Share
Hui Zhu, Peng Wu, Chenming Zhong, Shuming Li, Gang Lin, Xuefen Liu, Shuping Luo. Design and Synthesis of Donor-Acceptor-Donor (D-A-D) Type Aromatic Ketones for Photocatalytic C(sp3)—H Coupling Reactions[J]. Chinese Journal of Organic Chemistry, 2025, 45(9): 3441-3449.
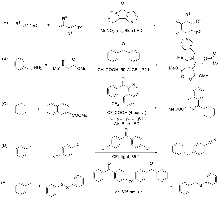

| Entry | Photocatalyst (mol%) | Solvent | Base | Time/h | Yieldb/% | |
|---|---|---|---|---|---|---|
| 1 | PC1 (20) | Toluene | NaHCO3 | 18 | 30 | |
| 2c | — | Toluene | NaHCO3 | 18 | None | |
| 3 | PC2 (20) | Toluene | NaHCO3 | 18 | 92 | |
| 4 | PC3 (20) | Toluene | NaHCO3 | 18 | 93 | |
| 5 | PC4 (20) | Toluene | NaHCO3 | 18 | 33 | |
| 6 | PC5 (20) | Toluene | NaHCO3 | 18 | 79 | |
| 7 | PC6 (20) | Toluene | NaHCO3 | 18 | 57 | |
| 8 | PC7 (20) | Toluene | NaHCO3 | 18 | 79 | |
| 9 | PC8 (20) | Toluene | NaHCO3 | 18 | 99 | |
| 10 | PC8 (5) | Toluene | NaHCO3 | 18 | 99 | |
| 11d | PC8 (5) | Toluene | NaHCO3 | 18 | None | |
| 12e | PC8 (5) | Toluene | NaHCO3 | 18 | None | |
| 13f | PC8 (5) | Toluene | NaHCO3 | 18 | None | |
| 14 | PC8 (5) | Toluene | NaHCO3 | 10 | 99 | |
| 15 | PC8 (5) | Toluene | K2HPO4 | 10 | 84 | |
| 16 | PC8 (5) | Toluene | CH3COONa | 10 | 23 | |
| 17 | PC8 (5) | Toluene | K2CO3 | 10 | 33 | |
| 18 | PC8 (5) | Toluene | Na2CO3 | 10 | 56 | |
| 19 | PC8 (5) | Toluene | NaHCO3 | 0.5 | 96 | |
| 20 | PC8 (5) | DMSO (0.1 mol/L) | NaHCO3 | 18 | None | |
| 21 | PC8 (5) | Hexane (0.1 mol/L) | NaHCO3 | 18 | Trace | |
| 22 | PC8 (5) | EtOAc (0.1 mol/L) | NaHCO3 | 18 | 7 | |
| 23 | PC8 (5) | MeCN (0.1 mol/L) | NaHCO3 | 18 | 32 | |
| 24 | PC8 (5) | MeCN (0.2 mol/L) | NaHCO3 | 18 | 55 | |
| 25 | PC8 (5) | MeCN (0.4 mol/L) | NaHCO3 | 18 | 99 | |
| 26 | PC8 (5) | MeCN (0.4 mol/L) | NaHCO3 | 0.5 | 87 | |
| Entry | Photocatalyst (mol%) | Solvent | Base | Time/h | Yieldb/% | |
|---|---|---|---|---|---|---|
| 1 | PC1 (20) | Toluene | NaHCO3 | 18 | 30 | |
| 2c | — | Toluene | NaHCO3 | 18 | None | |
| 3 | PC2 (20) | Toluene | NaHCO3 | 18 | 92 | |
| 4 | PC3 (20) | Toluene | NaHCO3 | 18 | 93 | |
| 5 | PC4 (20) | Toluene | NaHCO3 | 18 | 33 | |
| 6 | PC5 (20) | Toluene | NaHCO3 | 18 | 79 | |
| 7 | PC6 (20) | Toluene | NaHCO3 | 18 | 57 | |
| 8 | PC7 (20) | Toluene | NaHCO3 | 18 | 79 | |
| 9 | PC8 (20) | Toluene | NaHCO3 | 18 | 99 | |
| 10 | PC8 (5) | Toluene | NaHCO3 | 18 | 99 | |
| 11d | PC8 (5) | Toluene | NaHCO3 | 18 | None | |
| 12e | PC8 (5) | Toluene | NaHCO3 | 18 | None | |
| 13f | PC8 (5) | Toluene | NaHCO3 | 18 | None | |
| 14 | PC8 (5) | Toluene | NaHCO3 | 10 | 99 | |
| 15 | PC8 (5) | Toluene | K2HPO4 | 10 | 84 | |
| 16 | PC8 (5) | Toluene | CH3COONa | 10 | 23 | |
| 17 | PC8 (5) | Toluene | K2CO3 | 10 | 33 | |
| 18 | PC8 (5) | Toluene | Na2CO3 | 10 | 56 | |
| 19 | PC8 (5) | Toluene | NaHCO3 | 0.5 | 96 | |
| 20 | PC8 (5) | DMSO (0.1 mol/L) | NaHCO3 | 18 | None | |
| 21 | PC8 (5) | Hexane (0.1 mol/L) | NaHCO3 | 18 | Trace | |
| 22 | PC8 (5) | EtOAc (0.1 mol/L) | NaHCO3 | 18 | 7 | |
| 23 | PC8 (5) | MeCN (0.1 mol/L) | NaHCO3 | 18 | 32 | |
| 24 | PC8 (5) | MeCN (0.2 mol/L) | NaHCO3 | 18 | 55 | |
| 25 | PC8 (5) | MeCN (0.4 mol/L) | NaHCO3 | 18 | 99 | |
| 26 | PC8 (5) | MeCN (0.4 mol/L) | NaHCO3 | 0.5 | 87 | |


| Entry | PC | ELUMO/eV | EHOMO/eV | ΔE/eV | ΔES-T/eV |
|---|---|---|---|---|---|
| 1 | PC1 | -2.08 | -6.86 | 4.78 | 1.84 |
| 2 | PC2 | -2.76 | -7.76 | 5.00 | 2.06 |
| 3 | PC3 | -2.64 | -7.57 | 4.94 | 2.00 |
| 4 | PC8 | -2.50 | -6.92 | 4.42 | 1.48 |
| Entry | PC | ELUMO/eV | EHOMO/eV | ΔE/eV | ΔES-T/eV |
|---|---|---|---|---|---|
| 1 | PC1 | -2.08 | -6.86 | 4.78 | 1.84 |
| 2 | PC2 | -2.76 | -7.76 | 5.00 | 2.06 |
| 3 | PC3 | -2.64 | -7.57 | 4.94 | 2.00 |
| 4 | PC8 | -2.50 | -6.92 | 4.42 | 1.48 |
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
doi: 10.1039/d2sc04990b pmid: 36382292 |
| [22] |
|
| [23] |
|
| [24] |
doi: 10.7536/PC230306 |
| [25] |
doi: 10.1021/ja307861n pmid: 23025482 |
| [26] |
doi: 10.1021/acscatal.9b01284 |
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
doi: 10.1002/anie.201802656 pmid: 29566297 |
| [39] |
|
| [1] | Chenxu Jia, Conghao An, Jun Huang. Copper Catalyzed C—O Coupling for the Preparation of m-Phenoxybenzaldehyde [J]. Chinese Journal of Organic Chemistry, 2025, 45(9): 3412-3419. |
| [2] | Jinxia Li, Yuancheng Deng, Jiayu Li, Yihao Guo, Guangfeng Wang, Abing Duan, Shuanglin Qu. Mechanistic Study of Palladium-Catalyzed Ring-Opening/Cross-Coupling Reactions of Silacycles [J]. Chinese Journal of Organic Chemistry, 2025, 45(8): 2938-2944. |
| [3] | Qian Shi, Zhongyu Li, Han Li. Recent Advances in Photosensitizers of Heteroleptic Tris(cyclometalated) Iridium Complexes [J]. Chinese Journal of Organic Chemistry, 2025, 45(7): 2389-2405. |
| [4] | Jing Wang, Yuxun Chen, Jingjing Jiang. Study on Synthesis and Properties of New Indeno[2,1-b]fluorene-5,7-dione Derivatives [J]. Chinese Journal of Organic Chemistry, 2025, 45(7): 2444-2450. |
| [5] | Yongbo Tan, Hongbo Shu, Huawen Huang. Recent Advances in Photo-Induced Oxindole Formation from N-Arylacrylamides [J]. Chinese Journal of Organic Chemistry, 2025, 45(6): 2086-2108. |
| [6] | Yiming Du, Junsong Jia, Yulong Li, Wei Shu. Recent Progress on Catalytic Synthesis of Enantioenriched α-Arylated Ketones [J]. Chinese Journal of Organic Chemistry, 2025, 45(6): 1838-1870. |
| [7] | Lei Su, Xi Yang, Jie Yan, Yuanli Jiang, Lijuan Chen, Qingshu Zheng, Jiawang Liu. Recent Advances in Asymmetric Carbonylative Cross-Coupling Reactions [J]. Chinese Journal of Organic Chemistry, 2025, 45(6): 2007-2047. |
| [8] | Yan Yao, Niankai Fu. Recent Advances in Electrophotochemical Transition Metal Catalysis [J]. Chinese Journal of Organic Chemistry, 2025, 45(6): 1819-1837. |
| [9] | Shunxi Li, Lixu You, Yulong Li, Wei Shu. Recent Progress on Light-Mediated C—N Bond-Forming Processes from Ammonia and Equivalents [J]. Chinese Journal of Organic Chemistry, 2025, 45(5): 1460-1477. |
| [10] | Yu-Jia Chen, Zhi-Lin Liu, Kai Chen, Hao-Yue Xiang, Hua Yang. Metal-Free, Photo-Catalyzed Oxidation of Benzylic C—H Bonds to Access Carbonyl Functionality [J]. Chinese Journal of Organic Chemistry, 2025, 45(5): 1755-1762. |
| [11] | Chenyang Jiang, Yanli Yin, Zhiyong Jiang. Advances in Photoenzyme-Catalyzed Asymmetric Radical Addition Reactions [J]. Chinese Journal of Organic Chemistry, 2025, 45(5): 1614-1633. |
| [12] | Lihua Wu, Jianjing Yang, Kelu Yan, Lirong Xu, Jiangwei Wen. Research Progress of Single-Atom Photocatalysis in Organic Synthesis [J]. Chinese Journal of Organic Chemistry, 2025, 45(5): 1591-1613. |
| [13] | Lijing Niu, Chengjuan Wu, Wenjing Liang, Yan Geng, Yubin Dong. Construction of Covalent Organic Frameworks via Photocatalytic Cascade Reaction [J]. Chinese Journal of Organic Chemistry, 2025, 45(5): 1707-1715. |
| [14] | Genhong Zhang, Ruoxi Yu, Yuegang Chen. Research Progress in Photo/Electro-Induced C(sp2)—C(sp3) Bond Construction by Activating C—O Bond of Alcohols and Their Derivatives [J]. Chinese Journal of Organic Chemistry, 2025, 45(5): 1548-1568. |
| [15] | Fangfang Tan, Mengxin Shi, Wenmin Zhang, Yang Li. Photoinduced Catalysis for Biomass-Related Conversions [J]. Chinese Journal of Organic Chemistry, 2025, 45(5): 1523-1547. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||