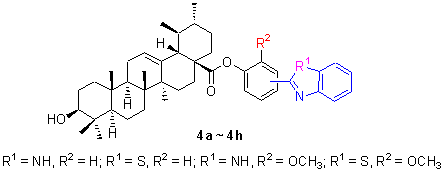
LI Cai-Hu
,
YANG Cong-Ling
,
ZHANG Kuan
,
SHI Wan-Qi
,
LI Jian-Zhong
,
LI Ying
,
YIN Shu-Fan
. Synthesis and Anti-inflammatory Activity of Novel Benzimidazole (Benzothiazole) Phenylursolate[J]. Chinese Journal of Organic Chemistry, 2012
, 32(01)
: 133
-137
.
DOI: 10.6023/cjoc1104011
[1] (a) Baricevic, D.; Sosa, S.; Della, R.; Tubaro, A.; Simonovska, B.; Krosna, A. J. Ethnopharmacol. 2001, 75, 125. (b) Liu, J. J. Ethnopharmacol. 1995, 49, 57.
[2] Yang, D.-J.; Li, Y.; Yin, S.-F. Chin. J. Org. Chem. 2008, 28, 1055 (in Chinese). (杨定菊, 李颖, 尹述凡, 有机化学, 2008, 28, 1055.)
[3] Ma, C.-M.; Cai, S.-Q.; Cui, J.-R.; Wang, R.-Q.; Tu, P.-F.; Hattori, M. Eur. J. Med. Chem. 2005, 40, 582.
[4] Tatsuzaki, J.; Taniguchi, M.; Bastow, K. F.; Nakagawa-Goto, K.; Morris-Natschke, S. L.; Itokawa, H. Bioorg. Med. Chem. 2007, 15, 6193.
[5] Huang, M. T.; Ho, C. T.; Wang, Z. Y.; Ferraro, T.; Lou, Y. R.; Stauber, K. Cancer Res. 1994, 54, 701.
[6] Saraswat, B.; Vises, P. K.; Agarwa, D. P. Phytother. Res. 2000, 14, 163.
[7] Gromovaya, V. F.; Shapoval, G. S.; Luik, A. I.; Averkov, A. I.; Lozinskii, M. O. Russ. J. Gen. Chem. 1997, 67(6), 942.
[8] Craigo, W. A.; Lesueur, B. W.; Skibo, E. B. J. Med. Chem. 1999, 42(17), 3324.
[9] Wells, G.; Bradshaw, T. D.; Diana, P.; Seaton, A.; Shi, D.-F.; Westwell, A. D.; Stevens, M. F. G. Bioorg. Med. Chem. Lett. 2000, 10, 513.
[10] Rana, A.; Siddiqui, N.; Khan, S. A. Indian J. Pharm. Sci. 2007, 69, 10.
[11] Chen, L.; Zhang, Y.-H.; Bi, X.-L.; Luo, Y.-Q. Central South Pharm. 2006, 12, 416 (in Chinese). (陈莉, 奕华, 毕小玲, 罗叶青, 中南药学, 2006, 12, 416.)
[12] Parish, R. C.; Stock, L. M. Tetrahedron Lett. 1964, 5(20), 1285.
[13] Yang, H.-J.; Hu, C.; Li, Y.; Yin, S.-F. Chin. J. Org. Chem. 2008, 28, 899 (in Chinese). (杨鸿均, 胡翠, 李颖, 尹述凡, 有机化学, 2008, 28, 899.)
[14] Kenny, R. S.; Mashelkar, U. C. J. Heterocycl. Chem. 2006, 43, 1367.
[15] Cramer, F.; Bär, H. P.; Rhaese, H. J.; Sänger, W.; Scheit, K. H.; Schneider, G.; Tennigkeit, J. Tetrahedron Lett. 1963, 4(16), 1039.