

Chinese Journal of Organic Chemistry >
Synthesis, Structures of Macrocyclic Compounds and Study on Recognition for La(Ⅲ) Ion
Received date: 2013-01-24
Revised date: 2013-03-15
Online published: 2013-04-03
Supported by
Project supported by the National Natural Science Foundation of China (No. 21061003) and the Natural Science Foundation of Guizhou Province (No.[2012]2151).
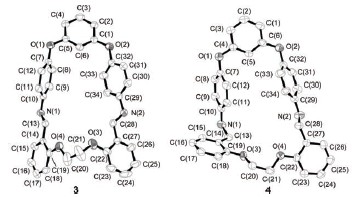
[1+1] Schiff base macrocyclic compound 3 has been synthesized from precursor 1 [1,2-bis(2'-formacyl- phenoxy)ethane] and precursor 2[2-resorcinol-bis(4-aminophenyl)ether] via condensation and cyclization using Ba2+ as template. The macrocycle 3 was further reduced giving macrocyclic compound 4, and the structures were characterized by 1H NMR, IR, MS and elemental analysis, respectively. The X-ray single crystal analysis reveals that the macrocycle 3 has a folding structure, and 4 shows a twisted unfolding structure. The study for the reaction of the macrocyclic compound 3 or 4 with a series of the rare earth ions is performed by UV-Vis absorption spectra technique, and the results show that the macrocycle 3 displayed a selective recognition for La3+ ion. Moreover, the JOB plot exhibited a 1:1 (host:guest) stoichiometry between the host 3 and La3+, and the binding constant (K) was determined using UV-vis titration method, K=7.59×103.
Key words: macrocyclic compound; crystal structure; La(Ⅲ) ion; recognition
Ou Min , Deng Yaxin , Wang Fangfang , Zhu Chun , Zhang Qilong , Zhu Bixue . Synthesis, Structures of Macrocyclic Compounds and Study on Recognition for La(Ⅲ) Ion[J]. Chinese Journal of Organic Chemistry, 2013 , 33(08) : 1798 -1803 . DOI: 10.6023/cjoc201301062
/
| 〈 |
|
〉 |