

Chinese Journal of Organic Chemistry >
Linear and Nonlinear Optical Properties of meso-Tetra(4-ferrocenylcarbonyloxyphenyl)porphyrin and Its Zinc(Ⅱ) Complex
Received date: 2013-08-28
Revised date: 2013-09-19
Online published: 2013-10-25
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21102068, 11174159) and the Natural Science Foundation of Inner Mongolia (No. 2013MS0207).
meso-Tetra(4-ferrocenylcarbonyloxyphenyl)porphyrin and its zinc(Ⅱ) complex were synthesized. Their linear and nonlinear optical properties were investigated using UV-Vis absorption spectra, steady state and time-resolved fluorescence spectra and open-aperture Z-scan measurements. The results indicated that the introduction of ferrocene to porphyrin had not affected the absorption spectra of porphyrin, but quenched the fluorescence intensively and shortened the fluorescence lifetimes of porphyrin, which indicated that there was almost no interaction between porphyrin and ferrocene in the ground state, but a strong interaction in the excited state. The reverse saturable absorptions of meso-tetra(4-ferrocenylcarbonyloxyphenyl)- porphyrin and its zinc(Ⅱ) complex were weaker than those of the corresponding meso-tetra(4-benzoyloxyphenyl)porphyrin and its zinc(Ⅱ) complex, but were stronger than that of fullerene C60 with excellent performance.
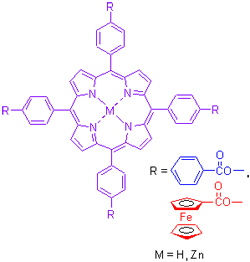
Key words: ferrocene; porphyrin; spectral property; Z-scan
Zhao Haiying , Gu Xuesong , Yan Xiaoqing , Liu Zhibo , Chen Qiang , Bian Zhanxi . Linear and Nonlinear Optical Properties of meso-Tetra(4-ferrocenylcarbonyloxyphenyl)porphyrin and Its Zinc(Ⅱ) Complex[J]. Chinese Journal of Organic Chemistry, 2014 , 34(2) : 371 -375 . DOI: 10.6023/cjoc201308036
[1] Zhang, X.-X.; Chen, C.-Y. Acta Chim. Sinica 2012, 70, 2475 (in Chinese).
(张现侠, 陈灿玉, 化学学报, 2012, 70, 2475.)
[2] Flores-Rojas, G. G.; Lijanova, I. V.; Morales-Saavedra, O. G.; Sanchez-Montes, K.; Martínez-García, M. Dyes Pigm. 2013, 96, 125.
[3] Huang, C.; Li, Y.; Song, Y.; Li, Y.; Liu, H.; Zhu, D. Adv. Mater. 2010, 22, 3532.
[4] Gonçalves, P. J.; Corrêa, D. S.; Franzen, P. L.; De Boni, L.; Almeida, L. M.; Mendonça, C. R.; Borissevitch, I. E.; Zílio, S. C. Spectrochim. Acta A 2013, 112, 309.
[5] Webster, S.; Odom, S. A.; Padilha, L. A.; Przhonska, O. V.; Peceli, D.; Hu, H.; Nootz, G.; Kachkovski, A. D.; Matichak, J.; Barlow, S.; Anderson, H. L.; Marder, S. R.; Hagan, D. J.; Van Stryland, E. W. J. Phys. Chem. B 2009, 113, 14854.
[6] Fungo, F.; Milanesio, M. E.; Durantini, E. N.; Otero, L.; Dittrich, T. J. Mater. Chem. 2007, 17, 2107.
[7] Straight, S. D.; Andréasson, J.; Kodis, G.; Moore, A. L.; Moore, T. A.; Gust, D. J. Am. Chem. Soc. 2005, 127, 2717.
[8] Fukuzumi, S.; Kojima, T. J. Mater. Chem. 2008, 18, 1427.
[9] Bi, C.; Li, Y.; Chen, H.; Yin, G.; Zhu, J. Chin. J. Chem. 2012, 30, 1722.
[10] Morales-Espinoza, E. G.; Lijanova, I. V.; Morales-Saavedra, O. G.; Torres-Zuñiga, V.; Hernandez-Ortega, S.; Martínez-García, M. Molecules 2011, 16, 6950.
[11] Kurotobi, K.; Osuka, A. Org. Lett. 2005, 7, 1055.
[12] Su, M.; Li, Q.; Wang, Y.-G.; Chen, S.-F.; Zhao, H.-Y.; Bian, Z.-X. Chin. J. Org. Chem. 2013, 33, 815 (in Chinese).
(苏敏, 李晴, 王亚光, 陈树峰, 赵海英, 边占喜, 有机化学, 2013, 33, 815.)
[13] Imahori, H.; Tamaki, K.; Guldi, D. M.; Luo, C.; Fujitsuka, M.; Ito, O.; Sakata, Y.; Fukuzumi, S. J. Am. Chem. Soc. 2001, 123, 2607.
[14] Zhao, H. Y.; Zhu, Y. Z.; Chen, C.; He, L.; Zheng, J. Y. Carbon 2012, 50, 4894.
[15] Yao, C.; Zhang, Y.; Yin, H.; Meng, Q.; Yu, C.; Li, J.; Yuan, P. Chem. Phys. Lett. 2013, 576, 35.
[16] Li, G.; Song, Y.; Hou, H.; Li, L.; Fan, Y.; Zhu, Y.; Meng, X.; Mi, L.; Inorg. Chem. 2003, 42, 913.
[17] Zou, Y.; Zhang, Q.; Hossain, A. M. S.; Li, S.; Wu, J.; Ke, W.; Jin, B.; Yang, J.; Zhang, S.; Tian, Y. J. Organomet. Chem. 2012, 720, 66.
[18] Yang, F.; Xu, X.; Gong, Y.; Qiu, W.; Sun, Z.; Zhou, J.; Audebert, P.; Tang, J. Tetrahedron 2007, 63, 9188.
[19] Tanihara, J.; Ogawa, K.; Kobuke, Y. J. Photochem. Photobiol. A 2006, 178, 140.
[20] Zhao, H. Y.; Chen, C.; Zhu, Y. Z.; Shi, M. Z.; Zheng, J. Y. J. Nanopart. Res. 2012, 14, DOI: 10.1007/s11051-012-0765-0.
[21] D’Souza, F.; Smith, P. M.; Gadde, S.; McCarty, A. L.; Kullman, M. J.; Zandler, M. E.; Itou, M.; Araki, Y.; Ito, O. J. Phys. Chem. B 2004, 108, 11333.
[22] Zang, N.; Dai, F.; Yan, W.-W.; Yuan, W.-J.; Zhu, Z.-A. Chin. J. Inorg. Chem. 2009, 25, 781 (in Chinese).
(臧娜, 戴放, 闫伟伟, 阮文娟, 朱志昂, 无机化学学报, 2009, 25, 781.)
[23] Zhang, X.-H.; Jiao, Z.; Yan, W.-W.; Yuan, W.-J.; Zhu, Z.-A. Acta Phys.-Chim. Sin. 2010, 26, 701 (in Chinese).
(张晓红, 矫志, 闫伟伟, 阮文娟, 朱志昂, 物理化学学报, 2010, 26, 701.)
[24] Wang, F.-F.; Zhang, K.; Zhu, B.-H.; Yan, Y.-L.; Gu, Y.-Z.; Qian, S.-X.; Guo, L.-J. Acta Opt. Sinica 2008, 28, 132 (in Chinese).
(王芳芳, 张琨, 朱宝华, 闫永丽, 顾玉宗, 钱士雄, 郭立俊, 光学学报, 2008, 28, 132.)
[25] Griesbeck, A. G.; Schäfer, M.; Uhlig, J. Adv. Synth. Catal. 2008, 350, 2104.
[26] Wang, R.-M.; Wang, Y.-P.; Lei, Z.-Q.; Feng, H.-Y.; Zhao, M.-Z. Northwest Normal Univ. (Nat. Sci.) 1994, 30, 98 (in Chinese).
(王荣民, 王云普, 雷自强, 冯罕玉, 赵明忠, 西北师范大学学报(自然科学版), 1994, 30, 98.)
/
| 〈 |
|
〉 |