

Chinese Journal of Organic Chemistry >
Secondary Metabolites and Antibacterial Activities of a Bruguiera sexangula var. Rhynchopetala-Derived Fungus Penicillium sp. (J41221)
Received date: 2014-01-09
Revised date: 2014-01-26
Online published: 2014-02-14
Supported by
Project supported by the Natural Science Foundation of Hainan Province (No. 213021), the National Natural Science Foundation of China (Nos. 81160391, 81360478, 21162009) and the National Undergraduate Innovation Training Programs (No. 2013116580).
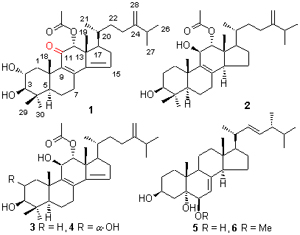
Under the guidance of bioassay, four tetracyclic triterpenoids and two steroids were isolated from the fermentation products of Penicillium sp. (J41221), a fungus obtained from a mangrove Bruguiera sexangula var. Rhynchopetala. Their stru- ctures were identified as 11-oxo-12α-acetoxy-4,4-dimethyl-24-methylene-5α-cholesta-8,14-diene-2α,3β-diol (1), 12α- acetoxy- 4,4-dimethyl-24-methylene-5α-cholesta-8-momoene-3β,11β-diol (2), 12α-acetoxy-4,4-dimethyl-24-methylene-5α-cholesta- 8,14-diene-3β,11β-diol (3), 12α-acetoxy-4,4-dimethyl-24-methylene-5α-cholesta-8,14-diene-2α,3β,11β-triol (4), cerevisterol (5) and (3β,5α,6β,22E)-6-methoxyergosta-7,22-diene-3,5-diol (6). Among them, compound 1 was a new naturally occurring compound, and 1 and 2 have no spectroscopic data reported until now. Compounds 2 and 4 showed inhibitory activities against Staphylococcus aureus, Escherichia coli and Micrococcus tetragenu, with minimum inhibitory concentration (MIC) values of 5 and 4.86 μmol/L, respectively.
Zheng Caijuan , Huang Guolei , Tang Xiongzhao , Wang Deneng , Gong Xiaolu , Zhang Qiang , Song Xiaoping , Chen Guangying . Secondary Metabolites and Antibacterial Activities of a Bruguiera sexangula var. Rhynchopetala-Derived Fungus Penicillium sp. (J41221)[J]. Chinese Journal of Organic Chemistry, 2014 , 34(6) : 1172 -1176 . DOI: 10.6023/cjoc201401014
[1] Blunt, J. W.; Copp, B. R.; Keyzers, R. A.; Munro, M. H. G.; Prinsep, M. R. Nat. Prod. Rep. 2013, 30, 237.
[2] Lin, P. Mangrove, Ocean Press, Beijing, 1984, p. 96 (in Chinese).
(林鹏, 红树林, 海洋出版社, 北京, 1984, p. 96.)
[3] Wang, Y.-S.; He, L.; Wang, Q.-J.; Zhang, S. Chin. J. Mar. Drugs 2004, 24, 26 (in Chinese).
(王友绍, 何磊, 王清吉, 张偲, 中国海洋药物, 2004, 24, 26.)
[4] Shao, C.-L.; Fu, X.-M.; Wang, C.-Y.; Han, L.; Liu, X.; Fang, Y.-C.; Liu, G.-X.; Guan, H.-S. J. Ocean Univ. China 2009, 39, 712 (in Chinese).
(邵长伦, 傅秀梅, 王长云, 韩磊, 刘新, 方玉春, 李国强, 刘光兴, 管华诗, 中国海洋大学学报, 2009, 39, 712.)
[5] Singh, S. B.; Zink, D.; Dombrowski, A. W.; Polishook, J. D.; Ondeyka, J. G.; Hirshfield, J.; Felock, P.; Hazuda, D. J. Bioorg. Med. Chem. 2003, 11, 1577.
[6] Singh, S. B. Tetrahedron Lett. 2000, 41, 6973.
[7] Yagen, B.; Horn, P.; Joffe, A. Z.; Cox, R. H. J. Chem. Soc., Perkin Trans. 1 1980, 2914.
[8] Ashe, B. M.; Fletcher, D. S. US 4874755, 1989 [Chem. Abstr. 1989, 4872727].
[9] Kawagishi, H.; Katsumi, R.; Sazawa, T.; Mizuno, T.; Hagiwara, T.; Nakamura, T. Phytochemistry 1988, 27, 2777.
[10] Ling, S. K.; Komorita, A.; Tanaka, T.; Fujioka, T.; Mihashi, K.; Kouno, I. J. Nat. Prod. 2002, 65, 656.
[11] Xiang, Z.-J.; Zhao, G.-R.; Yuan, J.-J.; Guo, Z.-X. Chin. Trad. Herb. Drugs 2006, 37, 211 (in Chinese). (向志军, 赵广荣, 元英进, 郭治昕, 中草药, 2006, 37, 211.)
[12] Solis, P. N.; Wright, C. W.; Anderson, M. M.; Gupta, M. P.; Phillipson, J. D. Planta Med. 1993, 59, 250.
[13] Meyer, B. N.; Ferrigni, N. R.; Putnam, J. E.; Jacobsen, L. B.; Nichols, D. E.; McLaughlin, J. L. Planta Med. 1982, 45, 31.
/
| 〈 |
|
〉 |