

Chinese Journal of Organic Chemistry >
Acetalization Catalyzed by Reusable N-Methylimidazole Trifluoromethyl Sulfonate
Received date: 2014-03-18
Revised date: 2014-03-30
Online published: 2014-04-05
Supported by
Project supported by the National Natural Science Foundation of China (No. 211720190).
A number of aldehydes and ketones are effectively converted to acetals in the presence of N-methylimidazole trifluoromethyl sulfonate. The catalyst can be readily recovered through filtration and reused with high activity.
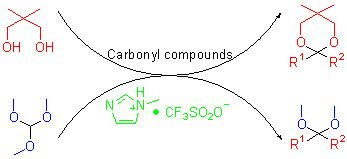
Key words: imidazole; carbonyl; acetalization
Liu Ningning , Chen Xi , Zhang Qingshan , Wu Qinpei . Acetalization Catalyzed by Reusable N-Methylimidazole Trifluoromethyl Sulfonate[J]. Chinese Journal of Organic Chemistry, 2014 , 34(7) : 1364 -1368 . DOI: 10.6023/cjoc201403042
[1] (a) Zhang, Q.; Deng, C.; Fang, L.; Xu, W.; Zhao, Q.; Zhang, J.; Wang, Y.; Lei, X. Chin. J. Chem. 2013, 31, 355.
(b) Meskens, F. A. J. Synthesis 1981, 501.
(c) Shen, Y. X.; Jiang H. F.; Wang, C. F. Chin. J. Org. Chem. 2008, 28, 782 (in Chinese).
(申艳霞, 江焕峰, 汪朝阳, 有机化学, 2008, 28, 782.)
(d) Liu, H.; Wu, Q.; Chen, X.; Xi, X.; Zhang, Q.; Li, Y. Carbohydr. Res. 2009, 344, 2342.
(e) Wu, Q.; Xi, X.; Chen, X.; Li, H.; Zhang, Q.; Li, Y. Chin. J. Chem. 2009, 27, 1962.
(f) Du, T.; Wu, Q.; Liu, H.; Chen, X.; Shu, Y.; Xi, X.; Zhang, Q.; Li, Y. Tetrahedron 2011, 67, 1096.
(g) Liu, N.; Yang, L.; Wang, J.; Chen, X.; Wu, Q. Chin. J. Org. Chem. 2014, DOI: 10.6023/cjoc201401038 (in Chinese).
(刘宁宁, 杨玲, 王金棒, 陈雪, 武钦佩, 有机化学, 2014, DOI: 10.6023/cjoc201401038.)
[2] (a) Johnson, W. S.; Harbert, C. A.; Stipanovic, R. D. J. Am. Chem. Soc. 1968, 90, 5279.
(b) Johnson, W. S.; Harbert, C. A.; Ratcliffe, B. E.; Stipanovic, R. D. J. Am. Chem. Soc. 1976, 98, 6188.
(c) Bartlett, P. A.; Johnson, W. S.; Elliott, J. D. J. Am. Chem. Soc. 1983, 105, 2088.
(d) Mori, A.; Yamamoto, H. J. Org. Chem. 1985, 50, 5444.
[3] (a) Gasparrini, F.; Giovannoli, M.; Misiti, D.; Palmieri, G. Tetrahedron 1984, 40, 1491.
(b) Yamada, Y.; Qiao, K.; Bao, Q.; Tomida, D.; Nagao, D.; Konno, M.; Yokoyama, C. Catal. Commun. 2009, 11, 227.
[4] (a) Williams, D. B. G.; Lawton, M. C. Green Chem. 2008, 10, 914.
(b) Ono, F.; Inatomi, Y.; Tada, Y.; Mori, M.; Sato, T. Chem. Lett. 2009, 38, 96.
[5] (a) Gopinath, R.; Haque, S. J.; Patel, B. K. J. Org. Chem. 2002, 67, 5842.
(b) Procuranti, B.; Connon, S. J. Org. Lett. 2008, 10, 4935.
[6] Ponde, D. E.; Deshpande, V. H.; Bulbule, V. J.; Sudalai, A. J. Org. Chem. 1998, 63, 1058.
[7] Qiao, Q.; Deng Y. Q. Acta Chim. Sinica 2002, 60, 528 (in Chinsese).
(乔焜, 邓友全, 化学学报, 2002, 60, 528.)
[8] Dutta Chowdhury, A.; Kumar Lahiri, G. Chem. Commun. 2012, 48, 3448.
[9] Mickelsen, K. J.; Tajc, C. M.; Greenwood, K. R.; Browder, C. C. Synth. Commun. 2011, 42, 186.
[10] (a) Zulfiqar, F.; Kitazume, T. Green Chem. 2000, 2, 296.
(b) Cole, A. C.; Jensen, J. L.; Ntai, I.; Tran, K. L. T.; Weaver, K. J.; Forbes, D. C.; Davis, J. H. J. Am. Chem. Soc. 2002, 124, 5962.
(c) Imrie, C.; Elago, E. R. T.; McCleland, C. W.; Williams, N. Green Chem. 2002, 4, 159.
[11] Doll, K. M.; Erhan, S. Z. Green Chem. 2008, 10, 712.
[12] Barbero, M.; Cadamuro, S.; Dughera, S.; Venturello, P. Synthesis 2008, 1379.
[13] Tayebee, R. Chin. J. Chem. 2008, 26, 2273.
[14] Li, D.; Shi, F.; Peng, J.; Guo, S.; Deng, Y. J. Org. Chem. 2004, 69, 3582.
[15] Shaterian, H. R.; Rigi, F. Chin. J. Chem. 2012, 30, 695.
[16] Madabhushi, S.; Mallu, K. K. R.; Chinthala, N.; Beeram, C. R.; Vangipuram, V. S. Tetrahedron Lett. 2012, 53, 697.
[17] Eshghi, H.; Rahimizadeh, M.; Saberi, S. Catal. Commun. 2008, 9, 2460.
[18] Hamada, N.; Kazahaya, K.; Shimizu, H.; Sato, T. Synlett 2004, 1074.
[19] Zou, J. Z.; Yi, F. P.; Zhang, L. R.; Wang, Z. Asian J. Chem. 2013, 25, 6643.
[20] Kumar, R.; Chakraborti, A. K. Tetrahedron Lett 2005, 46, 8319.
[21] Kumar, R.; Kumar, D.; Chakraborti, A. K. Synthesis 2007, 299.
[22] Clerici, A.; Pastori, N.; Porta, O. Tetrahedron 1998, 54, 15679.
[23] Lee, S. H.; Lee, J. H.; Yoon, C. M. Tetrahedron Lett 2002, 43, 2699.
[24] Zhu, W.; Li, Z. Z.; Yao, L. L.; Zhen, Z. B.; Zou, X. Z. Chin. J. Org. Chem. 2012, 32, 1146. (in Chinese).
(朱伟, 李忠洲, 姚璐璐, 郑祖彪, 邹新琢, 有机化学, 2012, 32, 1146.)
/
| 〈 |
|
〉 |