

Chinese Journal of Organic Chemistry >
An Efficient Synthesis of 2,3-Dihydrothiophene Derivatives from Four-Component Reactions under Catalyst-Free Conditions
Received date: 2014-04-03
Revised date: 2014-04-29
Online published: 2014-05-07
Supported by
Project supported by the National Natural Science Foundation of China (No. 21172188), and the Open Foundation of Jiangsu Key Laboratory of Green Synthetic Chemistry for Functional Materials (No. K201312).
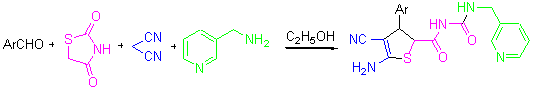
An efficient synthesis of 2,3-dihydrothiophene derivatives by the four-component reactions of aromatic aldehydes, thiazolidine-2,4-dione, malononitrile and pyridin-3-ylmethanamine has been reported. This reaction could be carried out smoothly without using any other catalysts in ethanol (95%) and the aromatic heterocyclic amine (pyridin-3-ylmethanamine) was first used for the synthesis of 2,3-dihydrothiophene derivatives. The advantages of this procure were mild reaction conditions, easy operation, and high yields. The products were identified by IR, 1H NMR and HRMS.
Zhang Jinpeng , Gao Yanian , Chen Ming , Jiang Ling , Xia Sheng , Rong Liangce . An Efficient Synthesis of 2,3-Dihydrothiophene Derivatives from Four-Component Reactions under Catalyst-Free Conditions[J]. Chinese Journal of Organic Chemistry, 2014 , 34(9) : 1895 -1899 . DOI: 10.6023/cjoc201404009
[1] Sperry Jeffrey, B.; Wright, D.L.Curr.Opin.Drug Discovery Dev.2005, 8, 723.
[2] Wu, C.; Decker, E.R.; Blok, N.; Bui, H.; You, T.J.; Wang, J.; Bourgoyne, A.R.; Knowles, V.; Berens, K.L.; Holland, G.W.; Brock, T.A.; Dixon, R.A.F.J.Med.Chem.2004, 47, 1969.
[3] Gewald, K.Angew.Chem.1961, 73, 114.
[4] Chakrabarti, J.K.; Hotten, T.M.; Tupper, D.E.Preparation of 2-Methylthienobenzodiazepine as Central Nervous System Agent (Lilly Industries Ltd., UK), Application, US, 11 pp, Cont-in-part of US Ser No 44, 844, abandoned.1997.
[5] Sun, J.; Zhang, L.L.; Xia, E.Y.; Yan, C.G.J.Org.Chem.2009, 74, 3398.
[6] Lu, G.P.; Zeng, L.Y.; Cai, C.Green Chem.2011, 13, 998.
[7] Tong, D.; Xia, S.; Tao, S.M.; Gao, L.J.; Feng, Y.J.; Rong, L.C.Res.Chem.Intermed.2013, 39, 561.
[8] Sun, J.; Xia, E.Y.; Yao, R.; Yan, C.G.Mol.Diversity 2011, 15, 115.
[9] Sun, J.; Wu, Q.; Xia, E.Y.; Yan, C.G.Synthesis 2010, 3987.
[10] Gao, L.J.; Xia, S.; Wu, N.; Tao, S.M.; Feng, Y.J.; Rong, L.C.Synth.Commun.2013, 43, 2590.
[11] Shi, D.Q.; Zou, Y.; Hu, Y.; Wu, H.J.Heterocycl.Chem.2011, 48, 896.
/
| 〈 |
|
〉 |