

Chinese Journal of Organic Chemistry >
Progress in Directed Transition Metal-Catalyzed Oxidation of Inactive C(sp3)—H Bonds
Received date: 2014-10-03
Revised date: 2014-10-05
Online published: 2014-12-08
Supported by
Project supported by the National Natural Science Foundation of China (No. 21261025), the Key Laboratory for the Chemistry and Molecular Engineering of Medicinal Resources, Ministry of Education (Nos. CMEMR2011-09, CMEMR2014-B08), and the Innovative Team & Outstanding Talent Program of Colleges and Universities in Guangxi.
The recent progress in transition metal-catalyzed oxidation of C(sp3)—H bonds has been made. Researches focus on the selective oxidation of methane, as well as the oxidation of methyl groups assisted by chelating directing groups. The researches lead to efficient and highly selective oxidative conversion that are unable to solve by traditional methods. This review covers the effect of directing groups (oxime, oxazoline, pyridine, amide, carboxylic acid, and hydroxyl) on promoting oxidation of C(sp3)—H bonds, the formation of acetylation, hydroxylation, carbonylation, esterification, and metal-catalyzed oxidative reaction system and mechanisms.
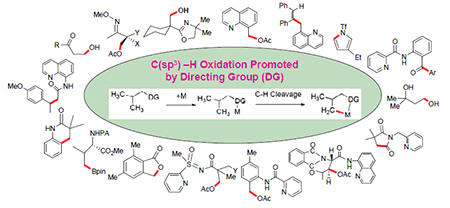
Key words: transition metal-catalyzed; inactive C(sp3)—H bonds; oxidation
Tan Mingxiong , Gu Yunqiong , Luo Xujian , Zhang Pei . Progress in Directed Transition Metal-Catalyzed Oxidation of Inactive C(sp3)—H Bonds[J]. Chinese Journal of Organic Chemistry, 2015 , 35(4) : 781 -788 . DOI: 10.6023/cjoc201409046
[1] Gutekunst, W. R.; Baran, P. S. Chem. Soc. Rev. 2011, 40, 1976.
[2] Chen, K.; Baran, P. S. Nature 2009, 459, 824.
[3] Xie, Y. X.; Song, R. J.; Xiang, J. N.; Li, J. H. Chin. J. Org. Chem. 2012, 32, 1555 (in Chinese).
(谢叶香, 宋仁杰, 向建南, 李金恒, 有机化学, 2012, 32, 1555.)
[4] Zhang, C. X.; Li, N. N.; Li, X.; Chang, H. H.; Liu, Q.; Wei, W. L. Chin. J. Org. Chem. 2014, 34, 81 (in Chinese).
(张聪霞, 李娜娜, 李兴, 常宏宏, 刘强, 魏文珑, 有机化学, 2014, 34, 81.)
[5] Feng, Y.; Chen, G. Angew. Chem., Int. Ed. 2010, 49, 958.
[6] Giannis, A.; Heretsch, P.; Sarli, V.; Stößel, A. Angew. Chem., Int. Ed. 2009, 48, 7911.
[7] Thomas, W. L.; Sanford, M. S. Chem. Rev. 2010, 110, 1147.
[8] Li, Y. M.; Ma, L. N.; Li, Z. P. Chin. J. Org. Chem. 2013, 33, 714 (in Chinese).
(李远明, 马丽娜, 李志平, 有机化学, 2013, 33, 704.)
[9] Shi, X. Y.; Han, X. Y.; Ma, W. J.; Wei, J. F. Chin. J. Org. Chem. 2011, 31, 297 (in Chinese).
(石先莹, 韩晓燕, 马文娟, 魏俊发, 有机化学, 2011, 31, 297.)
[10] Eegle, K. M.; Mei, T. S.; Wasa, M.; Yu, J. Q. Acc. Chem. Res. 2012, 67, 88.
[11] Chen, X.; Engle, K. M.; Wang, D. H.; Yu, J. Q. Angew. Chem., Int. Ed. 2009, 48, 5094.
[12] Chen, G. H.; Shi, Z. J. Nat. Sci. Rev. 2014, 2, 172.
[13] Crabtree, R. H. J. Organomet. Chem. 2004, 689, 4083.
[14] Hartwig, J. F. Chem. Soc. Rev. 2011, 40, 1992.
[15] White, M. C. Science 2012, 17, 806.
[16] Vedernikov, N. A. A. Chem. Res. 2012, 6, 803.
[17] Periana, R. A.; Taube, D. J.; Evitt, E. R.; LÖffler, D. G.; Wentrcek, P. R.; Voss, G.; Masuda, T. Science 1993, 259, 340.
[18] Periana, R. A.; Taube, D. J; Gamble, S.; Taube, H.; Satoh, T.; Fujii, H. Science 1998, 280, 560.
[19] Kao, L. C.; Hutson, A. C.; Sen, A. J. Am. Chem. Soc. 1991, 113, 700.
[20] Lin, M.; Shen, C.; Garcia-Zayas, E. A.; Sen, A. J. Am. Chem. Soc. 2001, 123, 1000.
[21] Bar-Nahum, I.; Khenkin, A. M.; Neumann, R. J. Am. Chem. Soc. 2004, 126, 10236.
[22] Periana, R. A.; Mironow, O.; Taube, D.; Bhalla, G.; Jones, C. Science 2003, 301, 814.
[23] Gretz, E.; Oliver, T. F.; Sen, A. J. Am. Chem. Soc. 1987, 109, 8109.
[24] An, Z. J.; Pan, X. l.; Liu, X. M.; Han, X. W.; Bao, X. H. J. Am. Chem. Soc. 2006, 128, 16028.
[25] Labinger, J. A.; Bercaw, J. E. Nature 2002, 41, 507.
[26] Rouquet, G.; Chatani, N. Angew. Chem., Int. Ed. 2013, 52, 11726.
[27] TranLy, D.; Daugulis, O. Angew. Chem., Int. Ed. 2012, 51, 5188.
[28] Carr, K.; Saxton, H. M.; Sutherland, J. K. J. Chem. Soc., Perkin Trans. 1 1988, 1599.
[29] Dangel, B. D.; Godula, K.; Youn, S. W.; Sezen, B.; Sames, D. J. Am. Chem. Soc. 2002, 124, 11856.
[30] Hinman, A.; Du, B. J. J. Am. Chem. Soc. 2003, 125, 11510.
[31] Renkema, K. B.; Kissin, Y. V.; Goldman, A. S. J. Am. Chem. Soc. 2003, 125, 7770.
[32] Chen, H.; Schlecht, S.; Semple, T. C.; Hartwig, J. F. Science 2000, 287, 1995.
[33] Bore, L.; Honda, T.; Gribble, G. W. J. Org. Chem. 2000, 65, 6278.
[34] Baldwin, J. E.; Jones, R. H.; Najera, C.; Yus, M. Tetrahedron 1985, 41, 699.
[35] Dick, A. R.; Hull, K. L.; Sanford, M. S. J. Am. Chem. Soc. 2004, 126, 2300.
[36] Desai, L. V.; Hull, K. L.; Sanford, M. S. J. Am. Chem. Soc. 2004, 126, 9542.
[37] Giri, R.; Liang, J.; Lei, J. G.; Li, J. J.; Wang, D. H.; Chen, X.; Naggar, I. C.; Guo, C. Y.; Foxman, B. M.; Yu, J. Q. Angew. Chem., Int. Ed. 2005, 44, 7420.
[38] Zhang, B.; Guan, H. X.; Liu, B.; Shi, B. F. Chin. J. Org. Chem. 2014, 34, 1487 (in Chinese).
(张博, 管晗曦, 刘斌, 史炳锋, 有机化学, 2014, 34, 1487.)
[39] Chen, M. S.; White, M. C. Science 2007, 318, 783.
[40] Newhouse, T.; Baran, P. S. Angew. Chem., Int. Ed. 2011, 50, 3362.
[41] McMurray, L.; ÒHara, F.; Gaunt, M. J. Chem. Soc. Rev. 2011, 40, 1885.
[42] Godula, K.; Sames, D. Science 2012, 312, 67.
[43] Ren, Z.; Mo, F. Y.; Dong, G. B. J. Am. Chem. Soc. 2012, 134, 16991.
[44] Zhang, J.; Khaskin, E.; Anderson, N. P.; Zavalij, P. Y.; Vedernikov, N. A. Chem. Commun. 2008, 3625.
[45] Wang, D. Y.; Zavalij, P. Y.; Vedernikov, N. A. Organometallics 2013, 32, 4882.
[46] Vedernikov, N. A. Chem. Commun. 2009, 4781.
[47] Liu, W. G.; Sberegaeva, A. V.; Nielsen, R. J.; Goddard, W. A.; Vedernikov, N. A. J. Am. Chem. Soc. 2014, 136, 2335.
[48] Sberegaeva, A. V.; Liu, W. G.; Nielsen, R. J.; Goddard, W. A.; Vedernikov, N. A. J. Am. Chem. Soc. 2014, 136, 4761.
[49] Liu, B. X.; Zhou,T.; Li, B.; Xu, S. S.; Song, H. B.; Wang, B. Q. Angew. Chem., Int. Ed. 2014, 53, 4191.
[50] Yang, M. Y.; Su, B.; Wang, Y.; Chen, K.; Jiang, X. Y.; Zhang, Y. F.; Zhang, X. S.; Chen, G. H.; Chen, Y.; Cao, Z. C.; Guo, Q. Y.; Wang, L. S.; Shi, Z. J. Nat. Commun. 2014, 5, 4707.
[51] Zhu, R. Y.; He, J.; Wang, X. C.; Yu, J. Q. J. Am. Chem. Soc. 2014, 136, 13194.
[52] Yan, J. X.; Li, H.; Liu, X. W.; Shi, J. L.; Wang, X.; Shi, Z. J. Angew. Chem., Int. Ed. 2014, 53, 4945.
[53] Zaitsev, V. G.; Shabashov, D.; Daugulis, O. J. Am. Chem. Soc. 2005, 127, 13154.
[54] Reddy, B. V. S.; Reddy, L. R.; Corey, E. J. Org. Lett. 2006, 15, 3391.
[55] Inoue, S.; Shiota, H.; Fukumoto, Y.; Chatani, N. J. Am. Chem. Soc. 2009, 131, 6898.
[56] Hasegawa, N.; Charra, V.; Inoue, S.; Fukumoto, Y.; Chatani, N. J. Am. Chem. Soc. 2011, 133, 8070.
[57] Zhang, L. S.; Chen, G. H.; Wang, X.; Guo, Q. Y.; Zhang, X. S.; Pan, F.; Chen, K.; Shi, S. J. Angew. Chem., Int. Ed. 2014, 53, 3899.
[58] Zhang, S. Y.; He, G.; Zhao, Y. S.; Wright, K.; Nack, W. A.; Chen, G. J. Am. Chem. Soc. 2012, 134, 7313.
[59] Xie, Y. J.; Yang, Y. Z.; Huang, L. H.; Zhang, X. B.; Zhang, Y. H. Org. Lett. 2012, 5, 1238.
[60] Cheng, T.; Yin, W. Y.; Zhang, Y.; Zhang, Y. N.; Huang, Y. Org. Biomol. Chem. 2014, 12, 1405.
[61] Rit, R. K.; Yadav, M. R.; Sahoo, A. K. Org. Lett. 2012, 14, 3724.
[62] Novák, P.; Correa, A.; Donaire, J. G.; Martin, R. Angew. Chem., Int. Ed. 2011, 50, 12236.
[63] Simmons, E. M.; Hartwig, J. F. Nature 2012, 483, 70.
[64] Majetich, G.; Wheless, K. Tetrahedron 1995, 51, 7095.
[65] Chen, K.; Richter, J. M.; Baran, P. S. J. Am. Chem. Soc. 2008, 130, 7247.
[66] Kasuya, S.; Kamijo, S.; Inoue, M. Org. Lett. 2009, 11, 3630.
/
| 〈 |
|
〉 |