

Chinese Journal of Organic Chemistry >
Microwave Assisted Solvent-Free Synthesis of 3-(Trifluoroacetyl)coumarins
Received date: 2014-11-08
Revised date: 2014-12-13
Online published: 2015-01-06
Supported by
Project supported by the Education Foundation of Henan Province (Nos.2009A150012, 2011A210018), the National Natural Science Foundation of China (No.21102037), and the Key Science and Technology Foundation of Henan Province (No.112101110200).
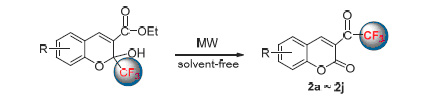
A series of 3-(trifluoroacetyl)coumarins were synthesized by recyclization of ethyl-2-hydroxy-2-trifluoromethyl-2H-chromene-3-carboxylates under microwave assisted solvent-free conditions. This method has the advantages of short reaction time, high yields (73%~88%), easy operation and environmental friendliness. The synthesized compounds were characterized by IR, NMR, HRMS and X-ray studies.
Key words: microwave; 3-(trifluoroacetyl)coumarins; recyclization; solvent-free
Yang Guoyu , Wang Caixi , Fan Sufang , Xie Puhui , Jin Qiu , Xu Cuilian . Microwave Assisted Solvent-Free Synthesis of 3-(Trifluoroacetyl)coumarins[J]. Chinese Journal of Organic Chemistry, 2015 , 35(5) : 1173 -1178 . DOI: 10.6023/cjoc201411012
[1] Sardari, S.; Mori, Y.; Horita, K.; Micetich, R. G.; Nishibe, S.; Daneshtalab, M. Bioorg. Med. Chem. 1999, 7, 1933.
[2] Abd El-Wahab, H.; Abd El-Fattah, M.; Abd El-Khalik, N.; Nassa H. S.; Abdelall, M. M. Prog. in Org. Coat. 2014,77, 1506.
[3] Puttaraju, K. B.; Shivashankar, K.; Chandra; Mahendra, M.; Rasal, V. P.; Vivek, P. N. V.; Rai, K.; Chanu, M. B. Eur. J. Med. Chem. 2013, 69, 316.
[4] Nasr, T.; Bondock, S.; Youns, M. Eur. J. Med. Chem. 2014, 76, 539.
[5] Sashidhara, K. V.; Modukuri, R. K.; Choudhary, D.; Rao, K. B.; Kumar, M.; Khedgikar, V.; Trivedi, R. Eur. J. Med. Chem. 2013, 70, 802.
[6] Kawate, T.; Iwase, N.; Shimizu, M.; Stanley, S. A.; Wellington, S.; Kazyanskaya, E.; Hung, D. T. Bioorg. Med. Chem. Lett. 2013, 23, 605.
[7] Zhang, Y.; Zou, B. Q.; Chen, Z. F.; Pan, Y. M.; Wang, H. S.; Liang, H.; Yi, X. H. Bioorg. Med. Chem. Lett. 2011, 21, 6811.
[8] Augustine, J. K.; Bombrun, A.; Ramappa, B.; Boodappa, C. Tetrahedron Lett. 2012, 53, 4422.
[9] Zareyee, D.; Serehneh, M. J. Mol. Catal. A: Chem. 2014, 391, 88.
[10] Kalita, P.; Kumar, R. Microporous. Mesopor. Mater. 2012, 149, 1.
[11] Patre, R. E.; Shet, J. B.; Parameswaran, P. S.; Tilve, S. G. Tetrahedron Lett. 2009, 50, 6488.
[12] Watson, B. T.; Christiansen, G. E. Tetrahedron Lett. 1998, 39, 6087.
[13] Song, A. M.; Wang, X. B.; Lam, K. S. Tetrahedron Lett. 2003, 44, 1755.
[14] Renuka, N.; Kumar, K. A. Bioorg. Med. Chem. Lett. 2013, 23, 6406.
[15] Patel, K. S.; Patel, J. C.; Dholariya, H. R.; Patel, K. D. Spectrochim. Acta A 2012, 96, 468.
[16] Xu, C. L.; Wan, X. S.; Dang, Y. L.; Su, H.; Jiang, Z. H.; Zhou, Z. F.; Wang, X. Y.; Deng, S. G. Chin. J. Henan Agric. Univ. 2009, 43, 100 (in Chinese). (徐翠莲, 宛新生, 党玉丽, 苏惠, 姜志红, 周占芳, 王喜云, 邓曙光, 河南农业大学学报, 2009, 43, 100.)
[17] Sun, Y. F.; Pan, W. L.; Zhang, D. D.; Lu, J. R.; Song, H. C. J. Zhongshan Univ. (Vat. Sci. Ed.) 2007, 46, 55 (in Chinese). (孙一峰, 许炎妹, 梁惠芬, 张东娣, 鲁敬荣, 潘文龙, 宋化灿, 中山大学学报(自然科学版), 2007, 46, 55.)
[18] Cheng, H. C.; Pei, Y.; Leng, F. Q.; Li, J. Y.; Liang, A. P.; Zou, D. P.; Wu, Y. J. Tetrahedron Lett. 2013, 54, 4483.
[19] Chizhov, D. L.; Sosnovskikh, V. Y.; Pryadeina, M. V.; Burgart, Y. V.; Saloutin, V. I.; Charushin, V. N. Synlett 2008, 281.
[20] Li, H. Q.; Cai, L.; Li, J. X.; Hu, Y. X.; Zhou, P. P.; Zhang J. M. Dyes Pigm. 2011, 91, 309.
[21] Shang, W. L.; Zhang, J. M.; Jin, J. A.; Chen, X. L. Chin. J. Org. Chem. 2011, 31, 1272 (in Chinese). (商文丽, 章建民, 金见安, 陈晓蕾, 有机化学, 2011, 31, 1272.)
[22] Li, B. Z.; Gu, Q.; Zhao, T. Q.; He, Y. H.; Tong, Y. N.; Zhang, Y. M. Chin. J. Org. Chem. 2012, 32, 1459 (in Chinese). (李宝志, 顾 强, 赵天琦, 贺元辉, 佟亚楠, 张玉敏, 有机化学, 2012, 32, 1459.)
[23] Li, L.; Liu, Q. J.; Sui, X. Y. Chin. J. Org. Chem. 2014, 34, 1669 (in Chinese). (李丽, 刘庆俭, 隋雪燕, 有机化学, 2014, 34, 1669.)
[24] Yang, G. Y.; Yang, J. T.; Wang, C. X.; Fan, S. F.; Xie, P. H.; Xu, C. L. J. Fluorine Chem. 2014, 168, 1.
[25] Xu, C. L.; Yang, G. Y.; Wang, C. X.; Fan, S. F.; Xie, L. X.; Gao, Y. Molecules 2013, 18, 11964.
/
| 〈 |
|
〉 |