

Chinese Journal of Organic Chemistry >
Magnetic Nanoparticle Supported Imino-pyridine Palladium Catalyzed Suzuki Reactions
Received date: 2015-07-16
Revised date: 2015-09-22
Online published: 2015-09-30
Supported by
Project supported by the Natural Science Foundation of Jiangsu Province (No. BK20150282) and the Scientific Research Foundation of Suzhou University of Science and Technology (No. XKQ201417).
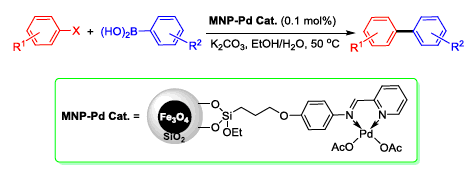
A new magnetic nanoparticle supported palladium catalyst was successfully prepared by attaching palladium acetates to imino-pyridine ligand functionalized silica-coated nano-Fe3O4. The as-prepared catalyst was characterized by element analysis, FTIR, TEM, XPS and ICP-AES. It was found to be an efficient catalyst for Suzuki reactions in aqueous medium. And the reactions of various aryl bromides with arylboronic acids could be carried out under air and low Pd loading conditions with 83%~98% yields. Moreover, the catalyst could be easily recovered by magnetic separation and reused five times without a significant loss of catalytic activity.
Key words: magnetic nanoparticle; palladium catalyst; Suzuki reaction; Schiff base
Zhang Qiang , Li Jihang , Zhao Xin . Magnetic Nanoparticle Supported Imino-pyridine Palladium Catalyzed Suzuki Reactions[J]. Chinese Journal of Organic Chemistry, 2016 , 36(1) : 130 -136 . DOI: 10.6023/cjoc201507013
[1] Miyaura, N.; Suzuki, A. Chem. Rev. 1995, 95, 2457.
[2] Corbet, J. P.; Mignani, G. Chem. Rev. 2006, 106, 2651.
[3] Fihri, A.; Bouhrara, M.; Nekoueishahraki, B.; Basset, J.-M.; Polshettiwar, V. Chem. Soc. Rev. 2011, 40, 5181.
[4] Tan, J. J.; Chen, Y. G.; Li, H. M.; Yasuda, N. J. Org. Chem. 2014, 79, 8871.
[5] Martin, R.; Buchwald, S. L. Acc. Chem. Res. 2008, 41, 1461.
[6] Fu, G. C. Acc. Chem. Res. 2008, 41, 1555.
[7] Chung, K. H.; So, C. M.; Wong, S. M.; Luk, C. H.; Zhou, Z.; Lau, C. P.; Kwong, F. Y. Chem. Commun. 2012, 48, 1967.
[8] Botella, L.; Nájera, C. Angew. Chem., Int. Ed. 2002, 41, 179.
[9] Alacid, E.; Nájera, C. Org. Lett. 2008, 10, 5011.
[10] Zhao, X. M.; Xiao, Z. Q.; Liang, T.; Gao, X.; Hao, X. Q.; Song, M. P. Chin. J. Org. Chem. 2014, 34, 2304 (in Chinese). (赵雪梅, 肖志强, 梁婷, 高翔, 郝新奇, 宋毛平, 有机化学, 2014, 34, 2304.)
[11] Tang, Y.; Yang, F. F.; Nie, S. P.; Wang, L.; Luo, Z. B.; Lu, H. F. Chin. J. Org.Chem. 2015, 35, 705 (in Chinese). (唐演, 杨飞飞, 聂士鹏, 王林, 罗治斌, 陆鸿飞, 有机化学, 2015, 35, 705.)
[12] Valente, C.; Çalimsiz, S.; Hoi, K. H.; Mallik, D.; Sayah, M.; Organ, M. G. Angew. Chem., Int. Ed. 2012, 51, 3314.
[13] Bai, L.; Wang, J. X. Adv. Synth. Catal. 2008, 350, 315.
[14] Ogasawara, S.; Kato, S. J. Am. Chem. Soc. 2010, 132, 4608.
[15] Wei, J. F.; Jiao, J.; Feng, J. J.; Lv, J.; Zhang, X. R.; Shi, X. Y.; Chen, Z. G. J. Org.Chem. 2009, 74, 6283.
[16] Chu, W. Y.; Wang, M.; Li, X. M.; Hou, Y. J.; Sun, Z. Z. Chin. J. Org. Chem. 2012, 32, 1666 (in Chinese).(初文毅, 王熳, 李新民, 侯艳君, 孙志忠, 有机化学, 2012, 32, 1666.)
[17] Rao, X. F.; Liu, C.; Zhang, Y. X.; Gao, Z. M.; Jin, Z. L. Chin. J. Catal. 2014, 35, 357 (in Chinese). (饶小峰, 刘春, 张义霞, 高占明, 金子林, 催化学报, 2014, 35, 357.)
[18] Zhang, C. Y.; Shi, R. B.; Chen, C. Y.; Jin, C. M. Chin. J. Org.Chem. 2013, 33, 611 (in Chinese). (张传越, 石若冰, 陈才元, 金传明, 有机化学, 2013, 33, 611.)
[19] Gawande, M. B.; Branco, P. S.; Varma, R. S. Chem. Soc. Rev. 2013, 42, 3371.
[20] Polshettiwar, V.; Luque, R.; Fihri, A.; Zhu, H. B.; Bouhrara, M.; Basset, J. M. Chem. Rev. 2011, 111, 3036.
[21] Feng, C. L.; Liu, J. P.; Gui, J. Z.; Liu, L. T. Chin. J. Appl. Chem. 2015, 32, 19 (in Chinese). (冯翠兰, 刘建平, 桂建舟, 刘澜涛, 应用化学, 2015, 32, 19.)
[22] Wang, Z.; Yu, Y.; Zhang, Y. X.; Li, S. Z.; Qian, H.; Lin, Z. Y. Green Chem. 2015, 17, 413.
[23] Li, P. H.; Wang, L.; Zhang, L.; Wang, G. W. Adv. Synth. Catal. 2012, 354, 1307.
[24] Du, Q. W.; Zhang, W.; Ma, H.; Zheng, J.; Zhou, B.; Li, Y. Tetrahedron 2012, 68, 3577.
[25] Beletskaya, I. P.; Cheprakov, A. V. Chem. Rev. 2000, 100, 3009.
[26] Wang, Y.; Wu, Z.; Wang, L.; Li, Z. K.; Zhou, X. G. Chem. Eur. J. 2009, 15, 8971.
[27] Lu, Y.; Shi, D. H.; You, Z. L.; Zhou, X. S.; Li, K. J. Coord. Chem. 2012, 65, 339.
[28] Dawood, K. M.; Kirschning, A. Tetrahedron 2005, 61, 12121.
[29] Phan, N. T. S.; Styring, P. Green Chem. 2008, 10, 1055.
[30] Dhara, K.; Sarkar, K.; Srimani, D.; Saha, S. K.; Chattopadhyay, P.; Bhaumik, A. Dalton Trans. 2010, 39, 6395.
[31] Jin, X. D.; Zhang, K. Y.; Sun, J.; Wang, J.; Dong, Z. P.; Li, R. Catal. Commun. 2012, 26, 199.
[32] Quali, A.; Laurent, R.; Caminade, A. M.; Majoral, J.-P.; Tailefer, M. J. Am. Chem. Soc. 2006, 128, 15990.
[33] Quali, A.; Spindler, J. F.; Jutand, A.; Tailefer, M. Adv. Synth. Catal. 2007, 349, 1906.
[34] Cloete, J.; Mapolie, S. F. J. Mol. Catal. A: Chem. 2006, 243, 221.
[35] Song, J.; Shen, Q.; Xu, F.; Lu, X. Tetrahedron 2007, 63, 5148.
[36] Trilla, M.; Pleixats, R.; Man, M. W. C.; Bied, C.; Moreau, J. E. Adv. Synth. Catal. 2008, 350, 577.
[37] Zhang, Q.; Su, H.; Luo, J.; Wei Y. Y. Green Chem. 2012, 14, 201.
[38] Demir, A. S.; Findik, H.; Saygili, N.; Subasi, N. T. Tetrahedron. 2010, 66, 1308.
[39] Schweizer, S.; Becht, J. -M.; Le Drian, C. Org. Lett. 2007, 9, 3777.
[40] Lipshutz, B. H.; Nihan, D. M.; Vinogradova, E.; Taft, B. R.; Boskovic, Z. V. Org. Lett. 2008, 10, 4279.
[41] Chung, J. W.; You, Y.; Huh, H. S.; An, B.-K.; Yoon, S.-J.; Kim, S. H.; Lee, S. W.; Park, S. Y. J. Am. Chem. Soc. 2009, 131, 8163.
[42] Qin, C. X.; Lu, W. J. J. Org. Chem. 2008, 73, 7424.
/
| 〈 |
|
〉 |