

Chinese Journal of Organic Chemistry >
Progress on the Synthesis of Enantiomerically Pure 3,4-Dihydropyrimidin-2-one Derivatives
Received date: 2015-07-01
Revised date: 2015-09-14
Online published: 2015-10-13
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21265009, 21362032).
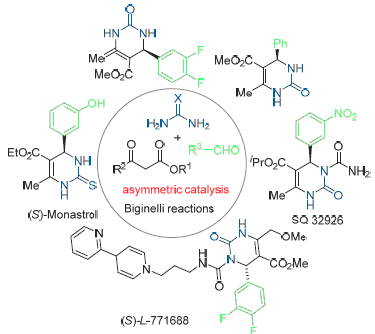
3,4-Dihydropyrimidinethiones are chiral molecules, however, only racemic products are isolated in the most reported Biginelli reactions. It has been proved that the absolute configuration of the C(4) stereogenic center has significant influence on the biological activity. The development in the accessing of optically active 3,4-dihydropyrimidinethiones focusing on the recent advances in the asymmetric catalytic Biginelli reactions is summarized.
Rao Honghong , Quan Zhengjun , Bai Lin , Ye Helin . Progress on the Synthesis of Enantiomerically Pure 3,4-Dihydropyrimidin-2-one Derivatives[J]. Chinese Journal of Organic Chemistry, 2016 , 36(2) : 283 -296 . DOI: 10.6023/cjoc201507001
[1] Biginelli, P. Gazz. Chim. Ital. 1893, 23, 360.
[2] Atwal, K. S.; Rovnyak, G. C.; Schwartz, J.; Moreland, S.; Hedberg, A.; Gougoutas, J. Z.; Malley, M. F.; Floyd, D. M. J. Med. Chem. 1990, 33, 1510.
[3] (a) Rovnyak, G. C.; Atwal, K. S.; Hedberg, A.; Kimball, S. D.; Moreland, S.; Gougoutas, J .Z.; O'Reilly, B. C.; Schwartz, J.; Malley, M. F. J. Med. Chem. 1992, 35, 3254. (b) Deres, K.; Schroder, C. H.; Paessens, A.; Goldmann, S.; Hacker, H. J.; Weber, O.; Kraemer, T.; Niewoehner, U.; Pleiss, U.; Stoltefuss, J.; Graef, E.; Koletzki, D.; Masantschek, R. N. A.; Reimann, A.; Jaeger, R.; Groâ, R.; Beckermann, B.; Schlemmer, K.-H.; Haebich, D.; Rubsamen-Waigmann, H. Science 2003, 299, 893. (d) Kappe, C. O. Eur. J. Med. Chem. 2000, 35, 1043. (c) Lewis, R. W.; Mabry, J.; Polisar, J. G.; Eagen, K. P.; Ganem, B.; Hess, G. P. Biochemistry 2010, 49, 4841. (d) Wan, J.-P.; Pan, Y. Mini-Rev. Med. Chem. 2012, 12, 337.
[4] Hurst, E. W.; Ann. N. Y. Ann. NY Acad. Sci. 1962, 98, 275.
[5] (a) Kappe, C. O. Tetrahedron 1993, 49, 6937. (b) Kappe, C. O. Acc. Chem. Res. 2000, 33, 879. (c) Wan, J.-P.; Liu, Y. Synthesis 2010, 2010, 3943.
[6] Atwal, K. S.; Swanson, B. N.; Unger, S. E.; Floyd, D. M.; Moreland, S.; Hedberg, A.; O'Reilly, B. C. J. Med. Chem. 1991, 34, 806.
[7] Barrow, J. C.; Nantermet, P. G.; Selnick, H. G.; Glass, K. L.; Rittle, K. E.; Gilbert, K. F.; Steele, T. G.; Homnick, C. F.; Freidinger, R. M.; Ransom, R. W.; Kling, P.; Reiss, D.; Broten, T. P.; Schorn, T. W.; Chang, R. S. L.; O′Malley, S. S.; Olah, T. V.; Ellis, J. D.; Barrish, A.; Kassahun, K.; Leppert, P.; Nagarathnam, D.; Forray, C. J. Med. Chem. 2000, 43, 2703.
[8] (a) Maliga, Z.; Kapoor, T. M.; Mitchison, T. J. Chem. Biol. 2002, 9, 989. (b) Debonis, S.; Simorre, J. P.; Crevel, I.; Lebeau, L.; Skoufias, D. A.; Blangy, A.; Ebel, C.; Gans, P.; Cross, R.; Hackney, D. D.; Wade, R. H.; Kozielski, F. Biochemistry 2003, 42, 338.
[9] (a) Du, B.-X.; Quan, Z.-J.; Da, Y.-X.; Zhang, Z.; Wang, X.-C. Adv. Synth. Catal. 2015, 357, 1270. (b) Quan, Z.-J.; Lv,Y.; Jing, F.-Q.; Jia, X.-D.; Huo, C.-D.; Wang, X.-C. Adv. Synth. Catal. 2014, 356, 325. (c) Quan, Z.-J.; Jing, F.-Q.; Zhang, Z.; Da, Y.-X.; Wang, X.-C. Eur. J. Org. Chem. 2013, 2013, 7175. (d) Quan, Z.-J.; Lv, Y.; Wang, Z.-J.; Zhang, Z.; Da, Y.-X.; Wang, X.-C. Tetrahedron Lett. 2013, 54, 1884. (e) Quan, Z.-J.; Zhang, Z.; Da, Y.-X.; Wang, X.-C. Chin. J. Org. Chem. 2009, 29, 876 (in Chinese). (权正军, 张彰, 达玉霞, 王喜存, 有机化学, 2009, 29, 876). (f) Wang, X. C.; Quan, Z. J.; Wang, F.; Wang, M. G.; Zhang, Z.; Li, Z. Synth. Commun. 2006, 36, 451.
[10] (a) Gong, L. Z.; Chen, X. H.; Xu, X.-Y. Chem. Eur. J. 2007, 13, 8920. (b) Heravi, M. M.; Asadi, S.; Lashkariani, B. M. Mol. Diversity 2013, 17, 389. (c) Wan, J.-P.; Lin, F.; Liu, Y. Curr. Org. Chem. 2014, 18, 687.
[11] Atwal, K. S.; Rovnyak, G. C.; Kimball, S. D.; Floyd, D. M.; Moreland, S.; Swanson, B. N.; Gougoutas, J. Z.; Schwartz, J.; Smillie, K. M.; Malley, M. F. J. Med. Chem. 1990, 33, 2629.
[12] Dondoni, A.; Massi, A.; Sabbatini, S. Tetrahedron Lett. 2002, 43, 5913.
[13] (a) Chartrain, C. M.; Ikemoto, N.; Taylor, C. S. WO 9907695, 1999 [Chem. Abstr. 1999, 130, 182478]. (b) Sidler, D. R.; Barta, N.; Li, W.; Hu, E.; Matty, L.; Ikemoto, N.; Campbell, J. S.; Chartrain, M.; Gbewonyo, K.; Boyd, R.; Corley, E. G.; Ball, R. G.; Larsen, R. D.; Reider, P. J. Can. J. Chem. 2002, 80, 646.
[14] Prasad, A. K.; Mukherjee, C.; Singh, S. K.; Brahma, R.; Singh, R.; Saxena, R. K.; Olsen, C. E.; Parmar, V. S. J. Mol. Catal. B: Enzyme 2006, 40, 93.
[15] Dondoni, A.; Massi, A.; Minghini, E.; Sabbatini, S.; Bertolasi, V. J. Org. Chem. 2003, 68, 6172.
[16] Dondoni, A.; Massi, A. Acc. Chem. Res. 2006, 39, 451.
[17] Kappe, C. O.; Uray, G.; Roschger, P.; Lindner, W.; Kratky, C.; Keller, W. Tetrahedron 1992, 48, 5473.
[18] Lou, S.; Taoka, B. M.; Ting, A.; Schaus, S. E. J. Am. Chem. Soc. 2005, 127, 11256.
[19] Lou, S.; Dai, P.; Schaus, S. E. J. Org. Chem. 2007, 72, 9998.
[20] Goss, J. M.; Schaus, S. E. J. Org. Chem. 2008, 73, 7651.
[21] Muñoz-Muñiz, O.; Juaristi, E. ARKIVOC 2003, xi, 16.
[22] Huang, Y.; Yang, F.; Zhu, C. J. Am. Chem. Soc. 2005, 127, 16386.
[23] Cai, Y;-F.; Yang, H.-M.; Li, L.; Jiang, K.-Z.; Lai, G.-Q.; Jiang, J.-X.; Xu, L.-W. Eur. J. Org. Chem. 2010, 2010, 4986.
[24] Karthikeyan, P.; Aswar, S. A.; Muskawar, P. N.; Bhagat, P. R.; Kumar, S. S. J. Organomet. Chem. 2013, 723, 154.
[25] (a) Stephen, J. C. Chem. Eur. J. 2006, 12, 5418. (b) Phillips A. M. F. Eur. J. Org. Chem. 2014, 2014, 7291.
[26] Chen, X.-H.; Xu, X.-Y.; Liu, H.; Cun, L.-F.; Gong, L.-Z. J. Am. Chem. Soc. 2006, 128, 14802.
[27] (a) Li, N.; Chen, X.-H.; Song, J.; Luo, S.-W.; Fan, W.; Gong, L.-Z. J. Am. Chem. Soc. 2009, 131, 15301. (b) Yu, J.; Shi, F.; Gong, L.-Z. Acc. Chem. Res. 2011, 44, 1156.
[28] Xu, F.; Huang, D.; Lin, X.; Wang, Y. Org. Biomol. Chem. 2012, 10, 4467.
[29] González-Olvera, R.; Demare, P.; Regla, I.; Juaristi, E. ARKIVOC 2008, vi, 61.
[30] Xin, J.; Chang, L.; Hou, Z.; Shang, D.; Liu, X.; Feng, X. Chem. Eur. J. 2008, 14, 3177.
[31] Li, Z. Y.; Xing, H. J.; Huang, G. L.; Sun, X. Q.; Jiang, J. L.; Wang, L. Y. Sci. China Chem. 2011, 54, 1726.
[32] Saha, S.; Moorthy, J. N. J. Org. Chem. 2011, 76, 396.
[33] Wu, Y.-Y.; Chai, Z.; Liu, X.-Y.; Zhao, G.; Wang, S.-W. Eur. J. Org. Chem. 2009, 904.
[34] Sohn, J.-H.; Choi, H.-M.; Lee, S.; Joung, S.; Lee, H.-Y. Eur. J. Org. Chem. 2009, 3858.
[35] (a) List, B.; Lerner, R. A.; Barbas, C. F. J. Am. Chem. Soc. 2000, 122, 2395. (b) Ahrendt, K. A.; Borths, C. J.; MacMillan, D. W. C. J. Am. Chem. Soc. 2000, 122, 4243. (c) Yang, D. Acc. Chem. Res. 2004, 37, 497. (d) Shi, Y. Acc. Chem. Res. 2004, 37, 488. (d) Wang, Y.; Han, R. G.; Zhao, Y. L.; Yang, S.; Xu, P. F. Dixon, D. J. Angew. Chem., Int. Ed. 2009, 48, 9834.
[36] Wang, Y.; Yang, H.; Yu, J.; Miao, Z.; Chen, R. Adv. Synth. Catal. 2009, 351, 3057.
[37] Wang, Y.; Yu, J.; Miao, Z.; Chen, R. Org. Biomol. Chem. 2011, 9, 3050.
[38] Frings, M.; Thomé, I.; Bolm, C. Beilstein J. Org. Chem. 2012, 8, 1443.
[39] Ding, D.; Zhao, C.-G. Eur. J. Org. Chem. 2010, 2010, 3802.
[40] Xu, D.-Z.; Li, H.; Wang, Y. Tetrahedron 2012, 68, 7867.
[41] An, D.; Fan, Y.-S.; Gao, Y.; Zhu, Z.-Q.; Zheng, L.-Y.; Zhang, S.-Q. Eur. J. Org. Chem. 2014, 2014, 301.
/
| 〈 |
|
〉 |