

Chinese Journal of Organic Chemistry >
Recent Progresses in the Chemistry of Münchnones
Received date: 2015-11-25
Revised date: 2015-12-21
Online published: 2016-01-21
Supported by
Project supported by the National Natural Science Foundation of China (No. 20834002) and the Natural Sciences Foundation of Tianjin City (No. 08JCZDJC21600).
Münchnones are mesoionic five-membered heterocycles, which play an important role in heterocycles as well as in organic chemistry. Since they were discovered by Huisgen in 1964, there has been growing interest in syntheses, properties and application of münchnones. One of the most significant properties of münchnones is that they can undergo cycloaddition reactions to unsaturated bonds as 1,3-dipoles. This reaction has become an important method for the synthesis of pyrroles and other heterocycles. Recently, remarkable progresses have been achieved in the synthesis of münchnones by means of organo-metallic reactions, the new reactive properties of münchnones have also been explored, and the application of münchnones in organic synthesis has been widely reported. In this review, these recent progresses in the chemistry of münchnone are summarized.
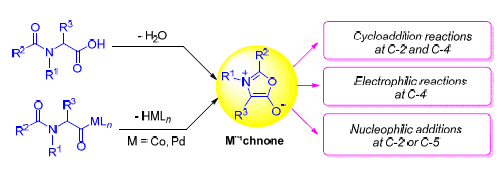
Key words: mü; nchones; mesoionic compounds; dipolar cycloaddition; heterocycles
Miao Qun , Sun Huailin . Recent Progresses in the Chemistry of Münchnones[J]. Chinese Journal of Organic Chemistry, 2016 , 36(5) : 913 -926 . DOI: 10.6023/cjoc201511047
[1] Miao, Q.; Sun, H.-L. Chin. Sci.Bull.2015, 60, 2003 (in Chinese).
(苗群, 孙怀林, 科学通报, 2015, 60, 2003. )
[2] Huisgen, R.; Gotthardt, H.; Bayer, H. O. Angew. Chem., Int. Ed.1964, 3, 136.
[3] Bayer, H. O.; Huisgen, R.; Knorr, R.; Schaefer, F. C. Chem. Ber.1970, 103, 2581.
[4] Potts, K. T.; Yao, S. J. Org. Chem.1979, 44, 977.
[5] Anderson, A. K.; Heider, A. R. Synth.Commun.1986, 16, 357.
[6] (a) Kato, H.; Tani, K.; Kurumisawa, H.; Tamura, Y. Chem.Lett.1980, 717.
(b) Kawase, M. J. Chem. Soc., Chem. Commun.1990, 1328.
[7] Huisgen, R.; Gotthardt, H.; Bayer, H. O.; Schaefer, F. C. Chem. Ber.1970, 103, 2611.
[8] (a) Gotthardt, H.; Huisgen, R.; Schaefer, F. C. Tetrahedron Lett.1964, 10, 487.
(b) Gotthardt, H.; Huisgen, R. Chem. Ber.1970, 103, 2625.
[9] (a) Turchi, I. J.; Dewar, M. J. S. Chem.Rev.1975, 75, 389.
(b) Turchi, I. J. Ind. Eng. Chem.Prod. Res. Dev.1981, 20, 32.
[10] Gingrich, H. L.; Baum, J. S. In Chemistry of Heterocyclic Compounds: Oxazoles,Vol. 45. Eds.: Turchi, I. J., Wiley, New York, 1986, pp. 731~922.
[11] Gribble, G. W. In Oxazoles: Synthesis, Reactions, and Spectroscopy,Vol. 60, Ed.: Palmer, D. C., Wiley, New York, 2003, pp. 473~534.
[12] Gribble, G. W. In Synthetic Applications of 1,3-Dipolar Cycloaddition Chemistry toward Heterocycles and Natural Products,Vol. 59, Eds.: Padwa, A.; Pearson. W. H., Wiley, New York, 2002, pp. 680~753.
[13] (a) Ollis, W. D.; Ramsden, C. A. Adv. Heterocycl. Chem.1976, 19, 1.
(b) Newton, C. G.; Ramsdem, C. A. Tetrahedron 1982, 38, 2965.
(c) Boyd, G. A. In Comprehensive Heterocyclic Chemistry, Vol. 6,Eds.: Katritzky, A. R.; Rees, C. W., Elsevier, Oxford, 1984, pp. 206~210.
(d) Ollis, W. D.; Stanforth, S. D.; Ramsdem, C. A. Tetrahedron 1985, 41, 2239.
(e) Kato, H.; Kobayashi, T. J. Synth.Org. Chem. Jpn.1990, 48, 672.
(f) Hartner, F. W. Jr. In Comprehensive Heterocyclic Chemistry II,Vol. 3, Ed.: Katritzky, A. R.; Rees, C. W.; Scriven, E. F. V., Elsevier, Oxford, 1996, pp. 284~286.
(g) Fortt, S. M. In Rodd's Chemistry of Carbon Compounds,Vol. IV C/D, Suppl. 2 to 2nd ed., Ed.: Sainsbury, M., Elsevier, Amsterdam, 1998, pp. 49~57.
(h) Gupta, R. R.; Kumar, M.; Gupta, V. Heterocyclic Chemistry II, Springer, Berlin, 1999, pp. 587~597.
(i) Yeh, V.; Iyengar, R. In Comprehensive Heterocyclic Chemistry III, Vol. 4, Eds: Katritzky. A. R.; Ramsden, C. A.; Scriven, E. F. V.; Taylor, R. J. K., Elsevier, Oxford, 2008, pp. 502~503.
(j) Lopchuk, J. M. In Metalation of Azoles and Related Five-Membered Ring Heterocycles, Eds.: Gribble, G. W., Springer, Berlin, 2012, pp. 399~402.
[14] Reissig, H-U.; Zimmer, R. Angew.Chem., Int. Ed.2014, 53, 9708.
[15] (a) Arndtsen, B. A. Chem. Eur. J.2009, 15, 302.
(b) Quesenel, J. S.; Arndtsen, B. A. Pure Appl.Chem.2013, 85, 377.
[16] (a) Dghaym, R. D.; Dhawan, R.; Arndtsen, B. A. Angew. Chem., Int. Ed.2001, 40, 3228.
(b) Bontemps, S.; Quesnel, J. S.; Worrall, K.; Arndtsen, B. A. Angew. Chem., Int. Ed.2011, 50, 8948.
(c) Worrall, K.; Xu, B.; Bontemps, S.; Arndtsen, B. A. J. Org.Chem.2011, 76, 170.
[17] Dhawan, R.; Dghaym, R. D.; Arndtsen, B. A. J. Am.Chem. Soc.2003, 125, 1474.
[18] Dhawan, R.; Arndtsen, B. A. J. Am.Chem. Soc.2004, 126, 468.
[19] Siamaki, A. R.; Arndtsen, B. A. J. Am.Chem. Soc.2006, 128, 6050.
[20] Dhawan, R.; Dghaym, R. D.; St Cyr, D. J.; Arndtsen, B. A. Org. Lett.2006, 8, 3927.
[21] Estévez, V.; Villacampa, M.; Menéndez, J. C. Chem. Soc. Rev.2010, 39, 4402.
[22] Siamaki, A. R.; Sakalauskas, M.; Arndtsen, B. A. Angew. Chem., Int. Ed.2011, 50, 6552.
[23] Leitch, D. C.; Kayser, L. V.; Han, Z. -Y.; Siamaki, A. R.; Keyzer, E. N.; Gefen, A.; Arndtsen, B. A. Nat.Commun.2015, 6, 7411.
[24] Lu, Y.; Arndtsen, B. A. Angew.Chem., Int. Ed.2008, 47, 5430
[25] (a) Sun, H.-L.; Zhang, J.; Liu, Q.-H.; Yu, L.; Zhao, J.-Y. Angew. Chem., Int. Ed.2007, 46, 6068.
(b) Zhang, Y.-P.; Sun, H.-L. Chem. J. Chin. Univ. 2014, 35, 54 (in Chinese).
(张永坡, 孙怀林, 高等学校化学学报, 2014, 35, 54.)
[26] Miao, Q. M.S. Thesis, Nankai University, Tianjin, 2015 (in Chinese).
(苗群, 硕士论文, 南开大学, 天津, 2015.)
[27] Lopchuk, J. M.; Gribble, G.. W. Tetrahedron Lett.2011, 52, 4106.
[28] Lopchuk, J. M.; Gribble, G.. W. Heterocycles 2011, 82, 1617.
[29] Pechenkin, S. Y.; Starosotnikov, A. M.; Bastrakov, M. A.; Kachala, V. V.; Shevelev, S. A. Mendeleev Commun.2012, 22, 35.
[30] Lopchuk, J. M.; Hughes, R. P.; Gribble, G.. W. Org. Lett.2013, 15, 5218.
[31] Lopchuk, J. M.; Song, M.; Butler, B.; Gribble, G. W. Synthesis 2015, 47, 2776.
[32] Grassi, G.; Foti, F.; Risitano, F.; Zona, D. Tetrahedron Lett.2005, 46, 1061.
[33] Bélanger, G..; April, M.; Dauphin, É.; Roy, S. J. Org. Chem.2007, 72, 1104.
[34] Cordaro, M.; Grassi, G.; Risitano, F.; Scala, A. Tetrahedron 2010, 66, 2713.
[35] (a) Cordaro, M.; Grassi, G.; Rescifina, A.; Chiacchio, U.; Risitano, F.; Scala, A. Tetrahedron 2011, 67, 608.
(b) Cordaro, M.; Grassi, G.; Rescifina, A.; Chiacchio, U.; Risitano, F.; Scala, A. J. Mol. Struct.2011, 991, 143.
[36] (a) Mazgarova, G. G.; Fatykhov, A. A.; Gataullin, R. R. Russ. J. Org. Chem.2014, 50, 1155.
(b) Mazgarova, G. G.; Fatykhov, A. A.; Gataullin R. R. Russ. J. Org. Chem.2014, 50, 1346.
[37] Gerster, H.; Maas, G. Z. Naturforsch.2008, 63b, 384.
[38] Harju, K.; Manevski, N.; Yli-Kauhaluoma, J. Tetrahedron 2009, 65, 9702.
[39] Fang, Y.; Larock, R. C.; Shi, F. Asian J. Org.Chem.2014, 3, 55.
[40] Lopchuk, J. M.; Gribble, G. W. Tetrahedron Lett.2014, 55, 2809.
[41] (a) Lira, B. F.; De Athayde Filho, P. F.; Miller, J.; Simas, A. M.; De Farias Dias, A.; Vieira, M. J. Molecules 2002, 7, 791.
(b) De Athayde Filho, P. F.; Miller, J.; Simas, A. M.; Lira, B. F.; De Souza Luis, J. A.; Zuckerman Schpector, J. Synthesis 2003, 685.
[42] Cantillo, D.; Ávalos, M.; Babiano, R.; Cintas, P.; Jiménez, J. L.; Light, M. E.; Palacios, J. C.; Rodríguez, V. Org. Biomol. Chem.2010, 8, 5367.
[43] Cantillo, D.; Ávalos, M.; Babiano, R.; Cintas, P.; Jiménez, J. L.; Light, M. E.; Palacios, J. C.; Porro, R. J. Org. Chem.2014, 79, 4201.
[44] Grassi, G.; Risitano, F.; Foti, F.; Cordaro, M.; Bruno, G.; Nicolò, F. Chem.Commun.2003, 1868.
[45] Kazhkennov, Z.-G. M.; Bush, A. A.; Babaev, E. V. Molecules 2005, 10, 1109.
[46] Kawase, M.; Koiwai, H.; Tanaka, T.; Tani, S.; Miyamae, H. Heterocycles 2001, 55, 1919.
[47] Saijo, R.; Hagimoto, Y.; Kawase, M. Org.Lett.2010, 12, 4776.
[48] Saijo, R.; Kawase, M. Tetrahedron Lett.2012, 53, 2782.
[49] Saijo, R.; Kurihara, K.; Akira, K.; Kawase, M. Heterocycles 2013, 87, 115.
[50] Saijo, R.; Kurihara, K.; Akira, K.; Uno, H.; Kawase, M. Tetrahedron Lett.2013, 54, 4418.
[51] Samanta, S. K.; Yli-Kauhaluoma, J. J. Comb. Chem.2005, 7, 142.
[52] Wang, J. K.; Zong, Y. X.; Yue, G. R. Synlett 2005, 1135.
[53] Gao, D.; Zhai, H.; Parvez, M.; Back, T. G. J. Org. Chem.2008, 73, 8057.
[54] Pinho e Melo, T. M. V. D.; Gomes, C. S. B.; Gonsalves, A. M.; Paixão, J. A.; Beja, A. M.; Silva, M. R.; Alte da Veiga, L. Tetrahedron 2002, 58, 5093.
[55] Pinho e Melo, T. M. V. D.; Soares, M. I. L.; Rocha Gonsavles, A. M. d'A.; Paixão, J. A.; Beja, A. M.; Silva, M. R.; Alte da Veiga, L.; Pessoa, J. C. J. Org.Chem.2002, 67, 4045.
[56] Lopes, S. M. M.; Laranjo, M.; Serra, A. C.; Abrantes. A. M.; d'A. Rocha Gonsavles, A. M.; Botelho, M. F.; Beja, A. M.; Silva, M. R.; Pinho e Melo, T. M. V. D. J. Heterocycl. Chem.2010, 47, 960.
[57] Dumitra?cu, F.; Caira, M. R.; Dr?ghici, B.; C?proiu, M. T.; Dumitrescu, D. G. Synlett2008, 813.
[58] Angle, S. R.; Qian, X. L.; Pletnev, A. A. Chin. J. Org.Chem.2007, 72, 2015.
[59] Pandey, P. S.; Rao, T. S. Bioorg. Med. Chem. Lett.2004, 14, 129.
[60] Park, W. K. C.; Kennedy, R. M.; Larsen, S. D.; Miller, S.; Roth, B. D.; Song, Y.; Steinbaugh, B. A.; Sun, K.; Tait, B. D.; Kowala, M. C.; Trivedi, B. K.; Auerbach, B.; Askew, V.; Dillon, L.; Hanselman, J. C.; Lin, Z.; Lu, G. H.; Robertson, A.; Sekerke, C. Bioorg. Med. Chem. Lett.2008, 18, 1151.
[61] Lopchuk, J. M.; Gribble, G. W. Tetrahedron Lett.2015, 56, 3208.
[62] Sugimoto, K.; Miyakawa, Y.; Tokuyama, H. Tetrahedron 2015, 71, 3619.
/
| 〈 |
|
〉 |