

Chinese Journal of Organic Chemistry >
Ce(SO4)2·4H2O Mediated Synthesis of Flavones and 2-Phenyl-4-quinolones
Received date: 2016-03-22
Revised date: 2016-06-20
Online published: 2016-07-08
Supported by
Project supported by the National Natural Science Foundation of China (No.31370370),the Science and Technology Planning Project of Hunan Pprovince (No.2013SK5077) and the Social Development of Science and Technology Project of Hainan Province (No.SF201419)
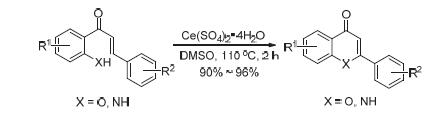
A simple and powerful procedure for the synthesis of flavones and quinolones was developed by using Ce(SO4)2·4H2O as oxidant, readily available 2'-hydroxychalcones and 2'-aminochalcones as raw materials, and dimethyl sulfoxide (DMSO) as solvent at 110℃. The new procedure is characterized with raw material easy to get, low manufacturing cost, mild reaction condi-tions and high yields.
Key words: Ce(SO4)2·; 4H2O; 2'-hydrochalcone; 2'-aminochalcone; flavone; quinolone
Liu Ruihuan , Wang Xuli , Cheng Fei , Li Fushuang , Xu Kangping , Tan Guishan . Ce(SO4)2·4H2O Mediated Synthesis of Flavones and 2-Phenyl-4-quinolones[J]. Chinese Journal of Organic Chemistry, 2016 , 36(11) : 2677 -2682 . DOI: 10.6023/cjoc201603036
[1] Silva, A. M. S.; Pinto, D. C. G. A.; Cavaleiro, J. A. S. Tetrahedron Lett. 1994, 35, 5899.
[2] Akama, T.; Shida, Y.; Sugaya, T.; Ishida, H.; Gomi, K.; Kasai, M. J. Med. Chem. 1996, 39, 3461.
[3] Alam, S.; Sarkar, Z.; Islam, A. J. Chem. Sci. 2004, 116, 29.
[4] Hans, N.; Grover, S. K. Synth. Commun. 1993, 23, 1021.
[5] Donnelly, J. A.; Farrell, D. F. J. Org. Chem. 1990, 55, 1757.
[6] Ahmed, N.; Ali, H.; van Lier, J. E. Tetrahedron Lett. 2005, 46, 253.
[7] Jung, S-H.; Cho, S-H.; Dang, T. H.; Lee, J-H.; Ju, J-H.; Kim, M-K.; Lee, S-H.; Ryu, J-C.; Kim, Y. Eur. J. Med. Chem. 2003, 38, 537.
[8] Du, Z.; Ng, H.; Zhang, K.; Zeng, H.; Wang, J. Org. Biomol. Chem. 2011, 9, 6930.
[9] Tang, E.; Chen, B.; Zhang, L.; Li, W.; Lin, J. Synlett 2011, 707.
[10] Ahmed, N.; van Lier, J. E. Tetrahedron Lett. 2007, 48, 13.
[11] Kim, D.; Ham, K.; Hong, S. Org. Biomol. Chem. 2012, 10, 7305.
[12] Wu, X.-F.; Neumann, H.; Beller, M. Chem. Eur. J. 2012, 18, 12595.
[13] Yatabe, T.; Jin, X.; Yamaguchi, K.; Mizuno, N. Angew. Chem., Int. Ed. 2015, 54, 13302.
[14] Ma, M.-L.; Li, M.; Gou, J.-J.; Ruan, T.-Y.; Jin, H.-S.; Zhang, L.-H.; Wu, L.-C.; Li, X.-Y.; Hu, Y.-H.; Zhao, Z. Bioorg. Med. Chem. 2014, 22, 6117.
[15] Huang, X.; Tang, E.; Xu, W.-M.; Cao, J. J. Comb. Chem. 2005, 7, 802.
[16] Guz, N. R.; Stermitz, F. R.; Johnson, J. B.; Beeson, T. D.; Willen, S.; Hsiang, J-F.; Lewis, K. J. Med. Chem. 2001, 44, 261.
[17] Lin, Y.; Zhou, Y.; Flavin, M. T.; Zhou, L.; Nie, W.; Chen, F. Bioorg. Med. Chem. 2002, 10, 2795.
[18] Hu, W.; Lin, J. P.; Song, L. R.; Long, Y. Q. Org. Lett. 2015, 17, 1268.
[19] An, Z.-Y.; Yan, Y.-Y.; Peng, D.; Ou, T.-M.; Huang, S.-L.; An, L.-K.; Gu, L.-Q.; Huang, Z.-S. Eur. J. Med. Chem. 2010, 45, 3895.
[20] Ding, D.; Li, X.; Wang, X.; Du, Y.; Shen, J. Tetrahedron Lett. 2006, 39, 6997.
[21] Huang, J.; Chen, Y.; King, A. O.; Dilmeghani, M.; Larsen, R. D.; Faul, M. M. Org. Lett. 2008, 10, 2609.
[22] Jones, C. P.; Anderson, K. W.; Buchwald, S. L. J. Org. Chem. 2007, 72, 7968.
[23] Sun, F.; Zhao, X.; Shi, D. Tetrahedron Lett. 2011, 52, 5633.
/
| 〈 |
|
〉 |