

Chinese Journal of Organic Chemistry >
Pepsin-Catalyzed Synthesis of 2,3-Dihydroquinazolin-4(1H)-one Derivatives
Received date: 2016-06-20
Revised date: 2016-09-17
Online published: 2016-10-18
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21462001, 21465002), the Program for Changjiang Scholars and Innovative Research Team in University (No. IRT13054), the Natural Science Foundation of Jiangxi Province (No. 20142BAB203008).
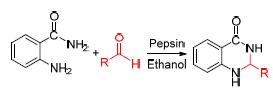
The catalytic property of some hydrolases for the cyclocondensation of aldehydes with 2-aminobenzamide was researched in alcohol solvent, and pepsin was selected as the best catalyst. The reaction conditions were optimized through investigating the temperature, the enzyme loading, the ratio of substrates and the reaction time. Under the optimal conditions, 5 mg of pepsin could catalyze the cyclizing reaction of aldehydes with 2-aminobenzamide, and the corresponding 2,3-dihydroquinazolin-4(1H)-ones were successfully obtained in high yields.
Xie Zongbo , Zhang Shiguo , Jiang Guofang , Liang Meng , Le Zhanggao . Pepsin-Catalyzed Synthesis of 2,3-Dihydroquinazolin-4(1H)-one Derivatives[J]. Chinese Journal of Organic Chemistry, 2017 , 37(2) : 514 -519 . DOI: 10.6023/cjoc201606030
[1] Abdollahi-Alibeik, M.; Shabani, E. Chin. Chem. Lett. 2011, 22, 1163.
[2] Abdel-Jalil, R. J.; Voelter, W.; Saeed, M. Tetrahedron Lett. 2004, 45, 3475.
[3] Shi, D.; Rong, L.; Wang, J.; Zhuang, Q.; Wang, X.; Hu, H. Tetrahedron Lett. 2003, 44, 3199.
[4] Shaabani, A.; Maleki, A.; Mofakham, H. Synth. Commun. 2008, 38 3751.
[5] Zong, Y.; Zhao, Y.; Luo, W.; Yu, X. H.; Wang, J. K.; Pan, Y. Chin. Chem. Lett. 2010, 21, 778.
[6] Ghashang, M.; Mansoor, S. S.; Aswin, K. Res. Chem. Intermed. 2015, 41, 3347
[7] Xua, B. L.; Chen, J. P.; Qiao, R. Z.; Fu, D. C. Chin. Chem. Lett. 2008, 19, 537.
[8] Zahed, K.-J.; Leila, Z. Acta Chim. Slovaca 2013, 60 178.
[9] Labade, V. B.; Shinde, P. V.; Shingare, M. S. Tetrahedron Lett. 2013, 54, 5578.
[10] (a) Shaterian, H. R.; Aghakhanizadeh, M. Res. Chem. Intermed. 2014, 40, 1655;
(b) Wang, J.; Zong, Y.; Fu, R.; Niu, Y.; Yue, G.; Quan, Z.; Wang, X.; Pan, Y. Ultrason. Sonochem. 2014, 21, 29;
(c) Davoodnia, A.; Allameh, S.; Fakhari, A. R.; Tavakoli-Hoseini, N. Chin. Chem. Lett. 2010, 21, 550;
(d) Safaei, H. R.; Shekouhy, M.; Shafiee, V.; Davoodi, M. J. Mol. Liq. 2013, 180, 139;
(e) Yassaghi, G.; Davoodnia, A.; Allameh, S.; Zare-Bidaki, A.; Tavakoli-Hoseini, N. Bull. Korean Chem. Soc. 2012, 33, 2724;
(f) Wang, S. L.; Yang, K.; Wang, X. S. Chin. J. Org. Chem. 2011, 31, 1235 (in Chinese).(王树良, 杨科, 王香善, 有机化学, 2011, 31, 1235.)
[11] Dabiri, M.; Salehi, P.; Otokesh, S.; Baghbanzadeh, M.; Kozehgary, G.; Mohammadi, A. A. Tetrahedron Lett. 2005, 46, 6123.
[12] Dabiri, M.; Salehi, P.; Baghbanzadeh, M.; Zolfigol, M. A.; Agheb, M.; Heydari, S. Catal. Commun. 2008, 9, 785.
[13] Abdollahi-Alibeik, M.; Shabani, E. J. Iranian Chem. Soc. 2014, 11, 351.
[14] Dhanunjaya Rao, A. V.; Vykunteswararao, B. P.; Bhaskarkumar, T.; Jogdand, N. R.; Kalita, D.; Lilakar, J. K. D.; Siddaiah, V.; Douglas Sanasi, P.; Raghunadh, A. Tetrahedron Lett. 2015, 56, 4714.
[15] Parthasaradhi, Y.; Rakhi, C.; Suresh, S.; Tangenda, S. J. Eur. J. Chem. 2013, 4, 462.
[16] Branneby, C.; Carlqvist, P.; Magnusson, A.; Hult, K.; Brinck, T.; Berglund, P. J. Am. Chem. Soc. 2003, 125 874.
[17] Chen, X.; Liu, B.-K.; Kang, H.; Lin, X.-F. J. Mol. Catal. B: Enzym. 2011, 68, 71.
[18] Guan, Z.; Fu, J.-P.; He, Y.-H. Tetrahedron Lett. 2012, 53, 4959.
[19] Purkarthofer, T.; Gruber, K.; Gruber-Khadjawi, M.; Waich, K.; Skranc, W.; Mink, D.; Griengl, H. Angew. Chem., Int. Ed. 2006, 45, 3454.
[20] Cai, J.-F.; Guan, Z.; He, Y.-H. J. Mol. Catal. B: Enzym. 2011, 68, 240.
[21] Mo, T.; Wang, J.; Gao, B. W.; Zhang, L.; Liu, X.; Wang, X. H.; Tu, P. F.; Shi, S. P. Chin. J. Org. Chem. 2015, 35, 1052 (in Chinese).(莫婷, 王娟, 高博闻, 张乐, 刘晓, 王晓辉, 涂鹏飞, 史社坡, 有机化学, 2015, 35, 1052.)
[22] Wu, Q.; Liu, B.-K.; Lin, X.-F. Curr. Org. Chem. 2010, 14, 1966.
[23] Humble, M. S.; Berglund, P. Eur. J. Org. Chem. 2011, 3391.
[24] Kapoor, M.; Gupta, M. N. Process Biochem. 2012, 47, 555.
[25] Safari, J.; Gandomi-Ravandi, S. J. Mol. Catal. A: Chem. 2014, 390, 1.
/
| 〈 |
|
〉 |