

Chinese Journal of Organic Chemistry >
Cross-Coupling of Directed C-H and Organometallic Reagents for C-C Bond Formation
Received date: 2017-03-27
Revised date: 2017-05-03
Online published: 2017-05-17
Supported by
Project supported by the National Basic Research Program of China (No. 2015CB856500) and the Natural Science Foundation of Tianjin City (No. 16JCYBJC20100).
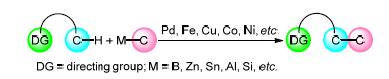
Transition metal-catalyzed C-H activation is one of the most important areas in organic synthesis. Directed C-H activation can obviate the prefunctionalization of substrate, therefore providing a highly efficient and concise strategy for C-C formation. The cross-coupling of transition metal-activated C-H bond with organic electrophilic reagents has been proven effective for construction of various C-C bonds. Meanwhile, the oxidative coupling between the corresponding intermediates with organometallic reagents has become the focus for chemists due to their high reactivity, and notable achievements have been made in recent years. Here the oxidative couplings of C-H bond and organometallic reagents have been discussed and summarized according to the hybridization of substrate and organometallic reagents.
Li Hua , Ren Xiangwei , Zhao Wentao , Tang Xiangyang , Wang Guangwei . Cross-Coupling of Directed C-H and Organometallic Reagents for C-C Bond Formation[J]. Chinese Journal of Organic Chemistry, 2017 , 37(9) : 2287 -2302 . DOI: 10.6023/cjoc201703036
[1] (a) Li, H.; Shi, Z.-J. Prog. Chem. 2010, 22, 1914(in Chinese). (李湖, 施章杰, 化学进展, 2010, 22, 1914.)
(b) Zhou, L.-H.; Lu, W.-J. Acta Chim. Sinica 2015, 73, 1250(in Chinese). (周励宏, 陆文军, 化学进展, 2015, 73, 1250.)
(c) Yu, J.-Q.; Ding, K.-L. Acta Chim. Sinica 2015, 73, 1223(in Chinese). (余金权, 丁奎岭, 化学学报, 2015, 73, 1223.)
(d) Zhu, Q.; Wang, L.; Xia, C.-G.; Liu, C. Chin. J. Org. Chem. 2016, 36, 2813(in Chinese). (朱庆, 王露, 夏春谷, 刘超, 有机化学, 2016, 36, 2813.)
[2] (a) Liu, C.; Liu, G.; Zhao, H. Chin. J. Chem. 2016, 34, 1048.
(b) Ren, X.; Kong, S.; Shu, Q.; Shu, M. Chin. J. Chem. 2016, 34, 373.
(c) Xu, J.-B.; Chen, P.-H.; Ye, J.-S.; Liu, G.-S. Acta Chim. Sinica 2015, 73, 1294(in Chinese). (徐佳斌, 陈品红, 叶金星, 刘国生, 化学学报, 2015, 73, 1294.)
[3] Sun, C.-L.; Li, B.-J.; Shi, Z.-J. Chem. Commun. 2010, 46, 677.
[4] Li, J.; Liu, K.; Duan, X.; Liu, J. Chin. J. Org. Chem. 2017, 37, 314(in Chinese). (李娟华, 刘昆明, 段新方, 刘晋彪, 有机化学, 2017, 37, 314.)
[5] Gao, K.; Yoshikai, N. Acc. Chem. Res. 2014, 47, 1208.
[6] Su, B.; Cao, Z.-C.; Shi, Z.-J. Acc. Chem. Res. 2015, 48, 886.
[7] Yang, Y.; Lan, J.; You, J. Chem. Rev. 2017, 117, 8787.
[8] (a) Dastbaravardeh, N.; Christakakou, M.; Haider, M.; Schnürch, M. Synthesis 2014, 46, 1421.
(b) Shang, M.; Sun, S.-Z.; Wang, H.-L.; Wang, M.-M.; Dai, H.-X. Synthesis 2016, 48, 4381.
[9] Negishi, E.; Anastasia, L. Chem. Rev. 2003, 103, 1979.
[10] Chen, M.; Zheng, X.; Li, W; He, J.; Lei, A. J. Am. Chem. Soc. 2010, 132, 4101.
[11] Molander, G. A.; Ellis, N. Acc. Chem. Res. 2007, 40, 275.
[12] Dick, G. R.; Woerly, E. M.; Burke, M. D. Angew. Chem., Int. Ed. 2012, 51, 2667.
[13] Giri, R.; Maugel, N.; Li, J.-J.; Wang, D.-H.; Breazzano, S. P.; Saunders, L. B.; Yu, J.-Q. J. Am. Chem. Soc. 2007, 129, 3510.
[14] Wasa, M.; Chan, K. S. L.; Yu, J.-Q. Chem. Lett. 2011, 40, 1004.
[15] Thuy-Boun, P. S.; Villa, G.; Dang, D.; Richardson, P.; Su, S.; Yu, J.-Q. J. Am. Chem. Soc. 2013, 135, 17508.
[16] Neufeldt, S. R.; Seigerman, C. K.; Sanford, M. S. Org. Lett. 2013, 15, 2302.
[17] Peng, P.; Wang, J.; Jiang, H.; Liu, H. Org. Lett. 2016, 18, 5376.
[18] Chen, Q.; Ilies, L.; Yoshikai, N.; Nakamura, E. Org. Lett. 2011, 13, 3232.
[19] Ilies, L.; Ichikawa, S.; Asako, S.; Matsubara, T.; Nakamura, E. Adv. Synth. Catal. 2015, 357, 2175.
[20] Graczyk, K.; Haven, T.; Ackermann, L. Chem.-Eur. J. 2015, 21, 8812.
[21] Negishi, E.; Okukado, N.; King, A. O.; Van Horn, D. E.; Spiegel, B. I. J. Am. Chem. Soc. 1978, 100, 3354.
[22] Xu, S.; Negishi, E. Acc. Chem. Soc. 2016, 49, 2158.
[23] Shang, R.; Ilies, L.; Nakamura, E. J. Am. Chem. Soc. 2015, 137, 7660.
[24] Chen, X.; Li, J.-J.; Hao, X.-S.; Goodhue, C.; Yu, J.-Q. J. Am. Chem. Soc. 2006, 128, 78.
[25] Cai, G.; Fu, Y.; Li, Y.; Wan, X.; Shi, Z. J. Am. Chem. Soc. 2007, 129, 7666.
[26] Feng, R.; Yao, J.; Liang, Z.; Liu, Z.; Zhang, Y. J. Org. Chem. 2013, 78, 3688.
[27] Tan, P.-W.; Haughey, M.; Dixon, D. J. Chem. Commun. 2015, 51, 4406.
[28] Laforteza, B. N.; Chan, K. S. L.; Yu, J.-Q. Angew. Chem., Int. Ed. 2015, 54, 11143.
[29] Xiao, K.-J.; Chu, L.; Chen, G.; Yu, J.-Q. J. Am. Chem. Soc. 2016, 138, 7796.
[30] Krasnov, V. P.; Gruzdev, D. A.; Levit, G. L. Eur. J. Org. Chem. 2012, 1471.
[31] Vogler, T.; Studer, A. Org. Lett. 2008, 10, 129.
[32] Chu, J.-H.; Tsai, S.-L.; Wu, M.-J. Synthesis 2009, 3757.
[33] Ilies, L.; Okabe, J.; Yoshikai, N.; Nakamura, E. Org. Lett. 2010, 12, 2838.
[34] Yoshikai, N.; Asako, S.; Yamakawa, T.; Ilies, L.; Nakamura, E. Chem.-Asian J. 2011, 6, 3059.
[35] Ilies, L.; Asako, S.; Nakamura, E. J. Am. Chem. Soc. 2011, 133, 7672.
[36] Shang, M.; Sun, S.-Z.; Wang, H.-L.; Wang, M.-M.; Dai, H.-X. Synthesis 2016, 48, 4381.
[37] Zaitsev, V. G.; Shabashov, D.; Daugulis, O. J. Am. Chem. Soc. 2005, 127, 13154.
[38] Shang, R.; Ilies, L.; Asako, S.; Nakamura, E. J. Am. Chem. Soc. 2014, 136, 14349.
[39] Gui, Q.; Chen, X.; Hu, L.; Wang, D.; Liu, J.; Tan, Z. Adv. Synth. Catal. 2016, 358, 509.
[40] Hu, L.; Gui, Q.; Chen, X.; Tan, Z.; Zhu, G. Org. Biomol. Chem. 2016, 14, 11070.
[41] Wang, D.; Yu, X.; Xu, X.; Ge, B.; Wang, X.; Zhang, Y. Chem. Eur. J. 2016, 22, 8663.
[42] Yu, X.; Wang, D.-S.; Xu, Z.; Yang, B.; Wang, D. Org. Chem. Front. 2017, 4, 1011.
[43] (a) Wasa, M.; Engle, K. M.; Yu, J.-Q. J. Am. Chem. Soc. 2009, 131, 9886.
(b) Wasa, M.; Engle, K. M.; Yu, J.-Q. J. Am. Chem. Soc. 2010, 132, 3680.
(c) Wasa, M.; Worrell, B. T.; Yu, J.-Q. Angew. Chem., Int. Ed. 2010, 49, 1275.
(d) Wasa, M.; Yu, J.-Q. Tetrahedron 2010, 66, 4811.
(e) Yoo, E. J.; Wasa, M.; Yu, J.-Q. J. Am. Chem. Soc. 2010, 132, 17378.
(f) Yoo, E.-J.; Ma, S.; Mei, T.-S. Chan, K. S. L. Yu, J.-Q. J. Am. Chem. Soc. 2011, 133, 7652.
[44] Wang, H.-W.; Cui, P.; Lu, Y.; Sun, W.-Y.; Yu, J.-Q. J. Org. Chem. 2016, 81, 3416.
[45] (a) Leow, D.; Li, G.; Mei, T.-S.; Yu, J.-Q. Nature 2012, 486, 518.
(b) Dai, H.-X.; Li, G.; Zhang, X.-G.; Stepan, A. F.; Yu, J.-Q. J. Am. Chem. Soc. 2013, 135, 7567.
[46] Wan, L.; Dastbaravardeh, N.; Li, G.; Yu, J-Q. J. Am. Chem. Soc. 2013, 135, 18056.
[47] Chinnagolla, R. K.; Jeganmohan, M. Org. Lett. 2012, 14, 5246.
[48] (a) Chinnagolla, R. K.; Jeganmohan, M. Chem. Commun. 2014, 50, 2442.
(b) Hubrich, J.; Himmler, T.; Rodefeld, L.; Ackermanna, L. Adv. Synth. Catal. 2015, 357, 474.
[49] Nishikata, T.; Abela, A. R.; Huang, S.; Lipshutz, B. H. J. Am. Chem. Soc. 2010, 132, 4978.
[50] Shang, M.; Sun, S.-Z.; Dai, H.-X.; Yu, J.-Q. Org. Lett. 2014, 16, 5666.
[51] (a) Zhou, H.; Xu, Y.-H.; Chung, W.-J.; Loh, T.-P. Angew. Chem., Int. Ed. 2009, 48, 5355.
(b) Li, W.; Yin, Z.; Jiang, X.; Sun, P. J. Org. Chem. 2011, 76, 8543.
[52] Zhao, S.; Liu, B.; Zhan, B.-B.; Zhang, W.-D.; Shi, B.-F. Org. Lett. 2016, 18, 4586.
[53] Kakiuchi, F.; Kan, S.; Igi, K.; Chatani, N.; Murai, S. J. Am. Chem. Soc. 2003, 125, 1698.
[54] Ueno, S.; Chatani, N.; Kakiuchi, F. J. Org. Chem. 2007, 72, 3600.
[55] Paymode, D. J.; Ramana, C. V. J. Org. Chem. 2015, 80, 11551.
[56] Tiwari, V. K.; Kamal, N.; Kapur, M. Org. Lett. 2017, 19, 262.
[57] Yang, Y.; Qiu, X.; Zhao, Y.; Mu, Y.; Shi, Z. J. Am. Chem. Soc. 2016, 138, 495.
[58] Wang, L.; Li, Z.; Qu, X.; Peng, W. Chin. J. Chem. 2015, 33, 1015.
[59] Wang, D.-H.; Mei, T.-S.; Yu, J.-Q. J. Am. Chem. Soc. 2008, 130, 17676.
[60] Engle, K. M.; Thuy-Boun, P. S.; Dang, M.; Yu, J.-Q. J. Am. Chem. Soc. 2011, 133, 18183.
[61] (a) Shabashov, D.; Daugulis, O. J. Am. Chem. Soc. 2010, 132, 3965.
(b) Samanta, R.; Antonchick, A. P. Angew. Chem., Int. Ed. 2011, 50, 5217.
(c) Yu, M.; Xie, Y.; Xie, C.; Zhang, Y. Org. Lett. 2012, 14, 2164.
(d) Wang, D.; Yu, X.; Yao, W.; Hu, W.; Ge, C.; Shi, X. Chem.-Eur. J. 2016, 22, 5543.
[62] Yao, J.; Yu, M.; Zhang, Y. Adv. Synth. Catal. 2012, 354, 3205.
[63] Wang, J.; Wang, S.; Wang, G.; Zhang, J.; Yu, X.-Q. Chem. Commun. 2012, 48, 11769.
[64] Wang, D.; Ge, B.; Li, L.; Shan, J.; Ding, Y. J. Org. Chem. 2014, 79, 8607.
[65] Ge, B.; Wang, D.; Dong, W.; Ma, P.; Li, Y.; Ding, Y. Tetrahedron Lett. 2014, 55, 5443.
[66] Wang, D.; Yu, X.; Ge, B.; Miao, H.; Ding, Y. Chin. J. Org. Chem. 2015, 35, 676.
[67] Liu, C.; Yang, F. Chin. J. Chem. 2016, 34, 1213.
[68] Xie, F.; Qi, Z.; Yu, S.; Li, X. J. Am. Chem. Soc. 2014, 136, 4780.
[69] Landge, V. G.; Midya, S. P.; Rana, J.; Shinde, D. R.; Balaraman, E. Org. Lett. 2016, 18, 5252.
[70] Arndtsen, B. A.; Bergman, R.; Mobley, T. A.; Peterson, T. Acc. Chem. Res. 1995, 28, 154.
[71] Zhao, J.-B.; Zhang, Q. Acta Chim. Sinica 2015, 73, 1235(in Chinese). (赵金钵, 张前, 化学学报, 2015, 73, 1235.)
[72] Chen, X.; Goodhue, C. E.; Yu, J.-Q. J. Am. Chem. Soc. 2006, 128, 12634.
[73] Wang, D.-H.; Wasa, M.; Giri, R.; Yu, J.-Q. J. Am. Chem. Soc. 2008, 130, 7190.
[74] Wasa, M.; Engle, K. M.; Lin, D. W.; Yoo, E. J.; Yu, J.-Q. J. Am. Chem. Soc. 2011, 133, 19598.
[75] (a) Bachrach, S. M. J. Org. Chem. 2008, 73, 2466.
(b) Li, S.; Zhu, R.-Y.; Xiao, K.-J.; Yu, J.-Q. Angew. Chem., Int. Ed. 2016, 55, 4317.
[76] Xiao, K.-J.; Lin, D. W.; Miura, M.; Zhu, R.-Y.; Gong, W.; Wasa, M.; Yu, J.-Q. J. Am. Chem. Soc. 2014, 136, 8138.
[77] Wang, X.; Yu, D.-G.; Glorius, F. Angew. Chem., Int. Ed. 2015, 54, 10280.
[78] (a) Pastine, S. J.; Gribkov, D. V.; Sames, D. J. Am. Chem. Soc. 2006, 128, 14220.
(b) Phani Kumar, N. Y.; Jeyachandran, R.; Ackermann, L. J. Org. Chem. 2013, 78, 4145.
(c) Dastbaravardeh, N.; Schnürch, M.; Mihovilovic, M. D. Org. Lett. 2012, 14, 1930.
[79] Spangler, J. E.; Kobayashi, Y.; Verma, P.; Wang, D.-H.; Yu, J.-Q. J.Am. Chem. Soc. 2015, 137, 11876.
[80] (a) He, G.; Zhao, Y.; Zhang, S.; Lu, C.-X.; Chen, G. J. Am. Chem. Soc. 2012, 134, 3.
(b) Zhang, S.-Y.; He, G.; Nack, W. A.; Zhao, Y.-S.; Li, Q.; Chen, G. J. Am. Chem. Soc. 2013, 135, 2124.
[81] Chan, K. S. L.; Wasa, M.; Chu, L.; Laforteza, B. N.; Miura, M.; Yu, J.-Q. Nat. Chem. 2014, 6, 146.
[82] Yuan, C.; Tu, G.; Zhao, Y. Org. Lett. 2017, 19, 356.
[83] He, J.; Takise, R.; Fu, H.; Yu, J.-Q. J. Am. Chem. Soc. 2015, 137, 4618.
[84] He, J.; Li, S.; Deng, Y.; Fu, H.; Laforteza, B. N.; Spangler, J. E.; Homs, A.; Yu, J.-Q. Science 2014, 343, 1216.
[85] He, C.; Gaunt, M. J. Angew. Chem., Int. Ed. 2015, 54, 15840.
[86] McNally, A.; Haffemayer, B.; Collins, B. S. L.; Gaunt, M. J. Nature 2014, 510, 129.
[87] Smalley, A. P.; Gaunt, M. J. J. Am. Chem. Soc. 2015, 137, 10632.
[88] Shang, R.; Ilies, L.; Matsumoto, A.; Nakamura, E. J. Am. Chem. Soc. 2013, 135, 6030.
[89] Gu, Q.; Al Mamari, H. H.; Graczyk, K.; Diers, E.; Ackermann, L. Angew. Chem., Int. Ed. 2014, 53, 3868.
[90] Ano, Y.; Tobisu, M.; Chatani, N. J. Am. Chem. Soc. 2011, 133, 12984.
[91] Luo, F-X.; Xu, X.; Wang, D.; Cao, Z.-C.; Zhang, Y.-F.; Shi, Z.-J. Org. Lett. 2016, 18, 2040.
[92] Luo, F.-X.; Cao, Z.-C.; Zhao, H.-W.; Wang, D.; Zhang, Y.-F.; Xu, X.; Shi, Z.-J. Organometallics 2017, 36, 18.
[93] (a) Zhu, Q.; Zhu, C.; Deng, Z.; He, G.; Chen, J.; Zhu, J.; Xia, H. Chin. J. Chem. 2016, 34, 1.
(b) Yuan, S.-T.; Wang, Y.-H.; Qiu, G.-Y.; Liu, J.-B. Chin. J. Org. Chem. 2017, 37, 566(in Chinese). (袁斯甜, 王艳华, 邱观音生, 刘晋彪, 有机化学, 2017, 37, 566.)
(c) Wang, M.-M; Wang, Z.-X; Shang, M.; Dai, H.-X. Chin. J. Org. Chem. 2015, 35, 570(in Chinese). (王明明, 王子萧, 商明, 戴辉雄, 有机化学, 2015, 35, 570.)
(d) Lu, B.-N.; Li, X.-Y.; Lin, Y.-M. Chin. J. Org. Chem. 2015, 35, 2275(in Chinese). (卢贝丽, 李现艳, 林咏梅, 有机化学, 2015, 35, 2275.)
/
| 〈 |
|
〉 |