

Chinese Journal of Organic Chemistry >
Semiregioselective Formation of Linaclotide with Orthogonal Cysteine Protection Strategy
Received date: 2017-04-12
Revised date: 2017-05-15
Online published: 2017-05-25
Supported by
Project supported by the State Key Laboratory of Toxicology and Medical Countermeasures of China (No. PMC201507) and the State Key Laboratory of Nuclear, Biological and Chemical Protection for Civilian (No. SKLNBC2013-01K).
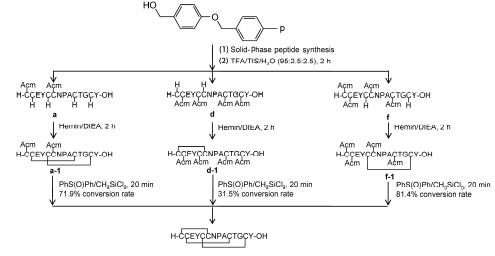
Six linear precursors of linaclotide containing different protected cysteine residues were synthesized by Fmoc solid-phase methods. Wang resin was used in the peptide syntheses. The protective groups of cysteine thiol were trityl (Trt) and acetamidomethyl (Acm) in the different positions. The six linear precursors of linaclotide include three[4 Trt+2 Acm] and three[2 Trt+4 Acm] ones. The linaclotide with three disulfide bonds was prepared from these linear precursors by semiregioselective strategy. Firstly, linear peptides were cleaved from Wang resins by TFA-TIS-H2O. At the same time, the Trt groups were removed to give free thiol groups, whereas Acm groups were still remained in the peptides. Secondly, the free thiol groups were oxidized by 20% hemin/DIEA system to from disulfide bond(s). Finally, cysteines containing Acm groups were deprotected by CH3SiCl3/PhS(O)Ph/TFA coaktail and disulfide bond(s) were formed simultaneously. The precursors of peptides[4 Trt(2,5,10,13)+2 Acm(1,6)],[2 Trt(1,6)+4 Acm(2,5,10,13)] and[2 Trt(5,13)+2 Acm(1,2,6,10)] give linaclotide at the conversion ratios of 71.9%, 31.5%, and 81.4% respectively. Other three peptides failed in the conversion or were found to be less suitable to prepare linaclotide. Our results indicated that the order of disulfide bond formation is very important to prepare linaclotide by using semiregioselective or regioselective strategies. The Cys5-Cys13 disulfide bond is the most privileged and should be formed firstly among the three disulfide bonds in linaclotide.
Key words: linaclotide; disulfide; oxidation; semiregioselective
Ge Weiwei , Chen Jing , Zhang Ye , Zong Liang , Zhang Ming , Dong Junjun . Semiregioselective Formation of Linaclotide with Orthogonal Cysteine Protection Strategy[J]. Chinese Journal of Organic Chemistry, 2017 , 37(9) : 2409 -2415 . DOI: 10.6023/cjoc201704020
[1] Cambridge, M. A. US 7304036, 2007.
[2] Harris, L. A.; Crowell, D. Curr. Opin. Mol. Ther. 2007, 9, 403.
[3] Andresen, V.; Camilleri, M. Drugs Future 2008, 33, 570.
[4] Wu Q. L.; Liu Z. G.; Fu C.; Lin Y. B.; Dai Q. Y. Chin. J. Org. Chem. 2010, 30, 1517(in Chinese). (吴巧玲, 刘珠果, 付超, 林原斌, 戴秋云, 有机化学, 2010, 30, 1517.)
[5] Eliasen, R.; Andresen, T. L.; Conde-Frieboes, K. W. Peptides 2012, 34, 144.
[6] Dekan, Z.; Mobli, M.; Pennington, M. W.; Fung, E.; Nemeth, E.; Alewood, P. F. Angew. Chem., Int. Ed. 2014, 53, 1.
[7] Veber, D. F.; Milkowski, J. D.; Varga, S. L.; Denkewalter, R. G.; Hirschmann, R. J. Am. Chem. Soc. 1972, 94, 5456.
[8] Munson, M. C.; Barany, G. J. Am. Chem. Soc. 1993, 115, 10203.
[9] Kawakami, T.; Aimoto, S. Tetrahedron Lett. 1998, 39, 7901.
[10] Hunter, M. J.; Komives, E. A. Anal. Biochem. 1995, 228, 173.
[11] Miriam, G. B.; Judit, T. P.; Marta, P. B.; Marta, P. B.; Oleg, W.;Matthieu, G.; Fernando, A. Biopolymers 2011, 96, 69.
[12] Ge, W. W.; Chen, J.; Zong, L.; Li, J.; Sui, S. H.; Wu, W. H.; Zhang, M.; Dong, J. J. Chem. J. Chin. Univ. 2017, 38, 1052(in Chinese). (葛巍巍, 陈静, 宗良, 李建, 隋少卉, 吴为辉, 张鸣, 董俊军, 高等学校化学学报, 2017, 38, 1052.)
[13] Akaji, K.; Tatsumi, T.; Yoshida, M.; Kimura, T.; Fujiwara, Y.; Kiso, Y. J. Am. Chem. Soc. 1992, 114, 4137.
[14] Ildikó, S.; Gitta, S.; Ference, H.; Gábor, M. Biopolymers 2006, 88, 20.
[15] Ge, W. W.; Chen, J.; Zhang, Y.; Zong, L.; Zhang, M.; Dong, J. J. J. Int. Pharm. Res. 2017, 44, 585(in Chinese). (葛巍巍, 陈静, 张也, 宗良, 张鸣, 董俊军, 国际药学研究杂志, 2017, 44, 585.)
/
| 〈 |
|
〉 |