

Chinese Journal of Organic Chemistry >
Recent Advances in Metal-Catalyzed 1,2-Difunctionalization of Conjugated Dienes
Received date: 2017-04-18
Revised date: 2017-05-22
Online published: 2017-05-25
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21232004, 21672142), the Program of Shanghai Subject Chief Scientists (No. 14XD1402300) and the Basic Research Foundation of Shanghai Science and Technology Committee (No. 15JC1402200).
The 1,2-difunctionalization of conjugated dienes is an important homogeneous catalytic reaction. The obtained products through 1,2-difunctionalization are widely existed in natural products and bioactive compounds, and are also sources of important organic intermediates, in addition, the preserved double bond in the difunctionalized product can be further transformed to give the desired structures or be functionalized sequentially to achieve multi-functionalization. The main difficulties focus on the encountered complex selectivities, including the regioselectivity, chemoselectivity and stereoselectivity in reactions. In recent years, with the development of organometallic chemistry, metal palladium, copper, iron or silver catalyzed 1,2-difunctionalizations of conjugated dienes have been reported in succession. In some cases the enantioselective 1,2-difunctionalizations of conjugated dienes were achieved via the introduction of chiral ligands. This review mainly focus on the recent metal catalyzed 1,2-difunctionalizations of conjugated dienes.
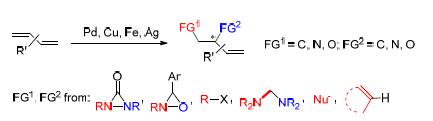
Key words: metal-catalyzed; conjugated diene; 1,2-difunctionalization
Wu Zhengxing , Zhang Wanbin . Recent Advances in Metal-Catalyzed 1,2-Difunctionalization of Conjugated Dienes[J]. Chinese Journal of Organic Chemistry, 2017 , 37(9) : 2250 -2262 . DOI: 10.6023/cjoc201704031
[1] (a) Kolb, H. C.; VanNieuwenhze, M. S.; Sharpless, K. B. Chem. Rev. 1994, 94, 2483.
(b) Bäckvall, J.-E. Modern Oxidation Methods, Wiley-VCH, Weinheim, 2004.
(c) Kotov, V.; Scarborough, C. C.; Stahl, S. S. Inorg. Chem. 2007, 46, 1910.
(d) Minatti, A.; Muñiz, K. Chem. Soc. Rev. 2007, 36, 1142.
(e) Chemler, S. R.; Fuller, P. H. Chem. Soc. Rev. 2007, 36, 1153.
(f) Jensen, K. H.; Sigman, M. S. Org. Biomol. Chem. 2008, 6, 4083.
(g) McDonald, R. I.; Liu, G.; Stahl, S. S. Chem. Rev. 2011, 111, 2981.
(h) Niu, F.; Nie, C.; Chen, Y.; Sun, X. Prog. Chem. 2014, 26, 1942(in Chinese). (牛凡凡, 聂昌军, 陈勇, 孙小玲, 化学进展, 2014, 26, 1942.)
(i) He, T.; Zeng, X. Chin. J. Org. Chem. 2017, 37, 798(in Chinese). (何天雄, 曾祥华, 有机化学, 2017, 37, 798.)
[2] (a) Smith, J. G. Synthesis 1984, 629.
(b) Tanner, D. Angew. Chem. Int. Ed. 1994, 33, 599.
(c) Kolb, H. C.; VanNieuwenhze, M. S.; Sharpless, K. B. Chem. Rev. 1994, 94, 2483.
(d) Ager, D. J.; Prakash, I.; Schaad, D. R. Chem. Rev. 1996, 96, 835.
(e) Bennani, Y. L.; Hanessian, S. Chem. Rev. 1997, 97, 3161.
(f) Lucet, D.; Gall, T. L.; Mioskowski, C. Angew. Chem. Int. Ed. 1998, 37, 2580.
(g) Gribble, G. W. Acc. Chem. Res., 1998, 31, 141.
(h) Bergmeier, S. C. Tetrahedron 2000, 56, 2561.
(i) Lauret, C. Tetrahedron:Asymmetry 2001, 12, 2359.
(j) Schneider, C. Synthesis 2006, 3919.
(k) Singh, G. S.; D'hooghe, M.; Kimpe, N. D. Chem. Rev. 2007, 107, 2080.
(l) Gao, Z.; Xiao, L.; Chen, J.; Xia, C. Chin. J. Catal. 2008, 29, 831(in Chinese). (高志文, 肖林飞, 陈静, 夏春谷, 催化学报, 2008, 29, 831.)
(m) Bataille, C. J. R.; Donohoe, T. J. Chem. Soc. Rev. 2011, 40, 114.
(n) Callebaut, G.; Meiresonne, T.; Kimpe, N. D.; Mangelinckx, S. Chem. Rev. 2014, 114, 7954.
(o) Wang, Q.; Chang, H.; Wei, W.; Liu, Q.; Gao, W.; Li, Y.; Li, X. Chin. J. Org. Chem. 2016, 36, 939(in Chinese). (王清宇, 常宏宏, 魏文珑, 刘强, 高文超, 李彦威, 李兴, 有机化学, 2016, 36, 939.)
[3] Prileschajew, N. Eur. J. Inorg. Chem. 1909, 42, 4811.
[4] Gilman, H. Organic Chemistry:An Advanced Treatise, Vol. 1, Wiley, New York, 1938, p. 36.
[5] (a) Criegee, R. Justus Liebigs Ann. Chem. 1936, 522, 75.
(b) Criegee, R. Angew. Chem. 1937, 50, 153.
[6] Kwart, H.; Kahn, A. A. J. Am. Chem. Soc. 1967, 89, 1950.
[7] (a) Sharpless, K. B.; Patrick, D. W.; Truesdale, L. K.; Biller, S. A. J. Am. Chem. Soc. 1975, 97, 2305.
(b) Chong, A. O.; Oshima, K.; Sharpless, K. B. J. Am. Chem. Soc. 1977, 99, 3420.
[8] (a) Bäckvall, J.-E. Tetrahedron Lett. 1978, 19, 163.
(b) Bäckvall, J.-E.; Björkman, E. E. J. Org. Chem. 1980, 45, 2893.
[9] (a) Sharpless, K. B.; Chong, A. O.; Oshima, K. J. Org. Chem. 1976, 41, 177.
(b) Hentges, S. G.; Sharpless, K. B. J. Am. Chem. Soc. 1980, 102, 4263.
(c) Katsuki, T.; Sharpless, K. B. J. Am. Chem. Soc. 1980, 102, 5974.
(d) Jacobsen, E. N.; Marko, I.; Mungall, W. S.; Schroeder, G.; Sharpless, K. B. J. Am. Chem. Soc. 1988, 110, 1968.
[10] (a) Aranyos, A.; Szabó, K. J.; Bäckvall, J.-E. J. Org. Chem. 1998, 63, 2523.
(b) Itami, K.; Palmgren, A.; Thorarensen, A.; Bäckvall, J.-E. J. Org. Chem. 1998, 63, 6466.
(c) Palmgren, A.; Larsson, A. L. E.; Bäckvall, J.-E. J. Org. Chem. 1999, 64, 836.
(d) Löfstedt, J.; Närhi, K.; Dorange, I.; Bäckvall, J.-E. J. Org. Chem. 2003, 68, 7243.
(e) Verboom, R. C.; Persson, B. A.; Bäckvall, J.-E. J. Org. Chem. 2004, 69, 3102.
(f) Piera, J.; Persson, A.; Caldentey, X.; Bäckvall, J.-E. J. Am. Chem. Soc. 2007, 129, 14120.
(g) Burks, H. E.; Kliman, L. T.; Morken, J. P. J. Am. Chem. Soc. 2009, 131, 9134.
(h) Schuster, C. H.; Li, B.; Morken, J. P. Angew. Chem. Int. Ed. 2011, 50, 7906.
[11] Xu, D.; Crispino, G. A.; Sharpless, K. B. J. Am. Chem. Soc. 1992, 114, 7571.
[12] O'Connor, J. M.; Stallman, B. J.; Clark, W. G.; Shu, A. Y. L.; Spada, R. E.; Stevenson, T. M.; Dieck, H. A. J. Org. Chem. 1983, 48, 807.
[13] (a) Larock, R. C.; Fried, C. A. J. Am. Chem. Soc. 1990, 112, 5882.
(b) Larock, R. C.; Berrios-Pena, N. G.; Narayanan, K. J. Org. Chem. 1990, 55, 3447.
[14] (a) Du, H.; Zhao, B.; Shi, Y. J. Am. Chem. Soc. 2007, 129, 762.
(b) Zhao, B.; Du, H.; Cui, S.; Shi, Y. J. Am. Chem. Soc. 2010, 132, 3523.
[15] Liao, L.; Jana, R.; Urkalan, K. B.; Sigman, M. S. J. Am. Chem. Soc. 2011, 133, 5784.
[16] Larock, R. C.; Harrison, L. W.; Hsu, M. H. J. Org. Chem. 1984, 49, 3662.
[17] Bar, G. L. J.; Lloyd-Jones, G. C.; Booker-Milburn, K. I. J. Am. Chem. Soc. 2005, 127, 7308.
[18] Houlden, C. E.; Bailey, C. D.; Ford, J. G.; Gagné, M. R.; Lloyd-Jones, G. C.; Booker-Milburn, K. I. J. Am. Chem. Soc. 2008, 130, 10066.
[19] Xing, D.; Yang, D. Org. Lett. 2013, 15, 4370.
[20] Cooper, S. P.; Booker-Milburn, K. I. Angew. Chem. Int. Ed. 2015, 54, 6496.
[21] (a) Kagechika, K.; Shibasaki, M. J. Org. Chem. 1991, 56, 4093.
(b) Kagechika, K.; Ohshima, T.; Shibasaki, M. Tetrahedron 1993, 49, 1773.
(c) Ohshima, T.; Kagechika, K.; Adachi, A.; Sodeoka, M.; Shibasaki, M. J. Am. Chem. Soc. 1996, 118, 7108.
[22] Du, H.; Yuan, W.; Zhao, B.; Shi, Y. J. Am. Chem. Soc. 2007, 129, 11688.
[23] Cornwall, R. G.; Zhao, B.; Shi, Y. Org. Lett. 2013, 15, 796.
[24] Stokes, B. J.; Liao, L.; de Andrade, A. M.; Wang, Q.; Sigman, M. S. Org. Lett. 2014, 16, 4666.
[25] Wu, X.; Lin, H.-C.; Li, M.-L.; Li, L. L.; Han, Z.-Y.; Gong, L.-Z. J. Am. Chem. Soc. 2015, 137, 13476.
[26] Liu, Y.; Xie, Y.; Wang, H.; Huang, H. J. Am. Chem. Soc. 2016, 138, 4314.
[27] Chen, S.-S.; Meng, J.; Li, Y.-H.; Han, Z.-Y. J. Org. Chem., 2016, 81, 9402.
[28] Chen, S.-S.; Wu, M.-S.; Han, Z.-Y. Angew. Chem., Int. Ed. 2017, 56, 6641.
[29] Yuan, W.; Du, H.; Zhao, B.; Shi, Y. Org. Lett. 2007, 9, 2589.
[30] Zhao, B.; Peng, X.; Cui, S.; Shi, Y. J. Am. Chem. Soc. 2010, 132, 11009.
[31] Zhao, B.; Peng, X.; Zhu, Y.; Ramirez, T. A.; Cornwall, R. G.; Shi, Y. J. Am. Chem. Soc. 2011, 133, 20890.
[32] Michaelis, D. J.; Ischay, M. A.; Yoon, T. P. J. Am. Chem. Soc. 2008, 130, 6610.
[33] Du, H.; Zhao, B.; Yuan, W.; Shi, Y. Org. Lett. 2008, 10, 4231.
[34] Zhao, B.; Du, H.; Shi, Y. J. Org. Chem. 2009, 74, 8392.
[35] Williamson, K. S.; Yoon, T. P. J. Am. Chem. Soc. 2010, 132, 4570.
[36] Williamson, K. S.; Yoon, T. P. J. Am. Chem. Soc. 2012, 134, 12370.
[37] (a) Llaveria, J.; Beltrán, Á.; Díaz-Requejo, M. M.; Matheu, M. I.; Castillón, S.; Pérez, P. J. Angew. Chem. Int. Ed. 2010, 49, 7092.
(b) Llaveria, J.; Beltrán, Á.; Sameera, W. M. C.; Locati, A.; Díaz-Requejo, M. M.; Matheu, M. I.; Castillón, S.; Maseras, F.; Pérez, P. J. J. Am. Chem. Soc. 2014, 136, 5342.
/
| 〈 |
|
〉 |