

Chinese Journal of Organic Chemistry >
Recent Advances in the Highly Stereoselective Synthesis of Tri-or Tetra-substituted Monofluoroalkenes
Received date: 2017-05-01
Revised date: 2017-06-28
Online published: 2017-07-07
Supported by
Project supported by the National Natural Science Foundation of China (No. 21472049).
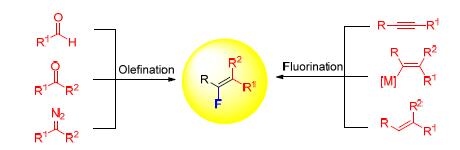
Monofluoroalkenes have found applications in many areas of research, including the design and development new materials and drug. The highly stereoselective synthesis of this privileged structural motif has attracted great synthetic attention. This review summarizes recent progresses in highly stereoselective synthesis of monofluoroalkenes from aldehydes, ketones and diazo compounds, other substrates such as alkynes, alkenyl metallic species and alkenes are also included. The advantages and disadvantages of different methods are discussed.
Liao Fumin , Yu Jinsheng , Zhou Jian . Recent Advances in the Highly Stereoselective Synthesis of Tri-or Tetra-substituted Monofluoroalkenes[J]. Chinese Journal of Organic Chemistry, 2017 , 37(9) : 2175 -2186 . DOI: 10.6023/cjoc201705001
[1] (a) Gelb, M. H. J. Am. Chem. Soc. 1986, 108, 3146.
(b) Damon, D. B.; Hoover, D. J. J. Am. Chem. Soc. 1990, 112, 6439.
(c) Erickson, J. A.; McLoughlin, J. I. J. Org. Chem. 1995, 60, 1626;
(d) Pesenti, C.; Viani, F. ChemBioChem 2004, 5, 590.
(e) He, Z.; Huang, Y,; Francis, V. Acta Chim. Sinica 2013, 71, 700(in Chinese) (何展荣, 黄毅勇, Francis, V., 化学学报, 2013, 71, 700.)
(f) Ni, C.; Zhu, L.; Hu, J. Acta Chim. Sinica 2015, 73, 90(in Chinese). (倪传法, 朱林桂, 胡金波, 化学学报, 2015, 73, 90.)
(g) Xiao, Y.; Pan, Q.; Zhang, X. Acta Chim. Sinica 2015, 73, 387(in Chinese). (肖玉兰, 潘强, 张新刚, 化学学报, 2015, 73, 387.)
(h) Zhang, K.; Xu, X.-H.; Qing, F.-L. Chin. J. Org. Chem. 2015, 35, 556(in Chinese). (张柯, 徐修华, 卿凤翎, 有机化学, 2015, 35, 556.)
(i) Rong, J.; Ni, C.; Wang, Y.; Kuang, C.; Gu, Y.; Hu, J. Acta Chim. Sinica 2017, 75, 105(in Chinese). (荣健, 倪传法, 王云泽, 匡翠文, 顾玉诚, 胡金波, 化学学报, 2017, 75, 105.)
[2] (a) Hiyama, T. In Organofluorine Compounds:Chemistry and Ap-plications, Ed.:Yamamoto, H., Springer-Verlag, Berlin, 2000.
(b) Fluorine in Medicinal Chemistry and Chemical Biology, Ojima, I. Ed.; Wiley-Blackwell:United Kingdom, 2009. For reaviews:
(c) Bégué, J.-P.; Bonnet-Delphon, D. J. Fluorine Chem. 2006, 127, 992.
(d) Kirk, K. L. J. Fluorine Chem. 2006, 127, 1013.
(e) Müller, K.; Faeh, C.; Diederich, F. Science 2007, 317, 1881.
(f) Hagmann, W. K. J. Med. Chem. 2008, 51, 4359.
(g) Purser, S.; Moore, P. R.; Swallow, S.; Gouverneur, V. Chem. Soc. Rev. 2008, 37, 320.
(h) Jeschke, P. ChemBioChem 2004, 5, 570.
(i) Pagliaro, M.; Ciriminna, R. J. Mater. Chem. 2005, 15, 4981.
(j) Ni, C.; Hu, J. Chem. Soc. Rev. 2016, 45, 5441.
[3] Thayer, A. M. Chem. Eng. News 2006, 84, 15.
[4] (a) Osada, S.; Sano, S.; Ueyama, M.; Chuman, Y.; Kodama, H.; Sakaguchi, K. Bioorg. Med. Chem. 2010, 18, 605.
(b) Malo-Forest, M.; Landelle, G.; Roy, J.-A.; Lacroix, J.; Gaudreault, R.-C.; Paquin, J.-F. Bioorg. Med. Chem. Lett. 2013, 23, 1712.
(c) Dutheuil, G.; Couve-Bonnaire, S.; Pannecoucke, X. Angew. Chem. Int. Ed. 2007, 46, 1290.
(d) Wong, O. A.; Shi, Y. A. J. Org. Chem. 2009, 74, 8377.
(e) Guérin, D.; Gaumont, A.-C.; Dez, I.; Mauduit, M.; Couve-Bonnaire, S.; Pannecoucke, X. ACS Catal. 2014, 4, 2374.
(f) Dai, W.; Xiao, J.; Jin, G.; Wu, J.; Cao, S. J. Org. Chem. 2014, 79, 10537.
[5] (a) Shen, Q.; Huang, Y.-G.; Liu, C.; Xiao, J.-C.; Chen, Q.-Y.; Guo, Y. J. Fluorine Chem. 2015, 179, 14.
(b) Tian, P.; Feng, C.; Loh, T.-P. Nat. Commun. 2015, 6, 7472.
(c) Zhang, X.; Lin, Y.; Zhang, J.; Cao, S. RSC Adv. 2015, 5, 7905.
(d) Wu, J.; Xiao, J.; Dai, W.; Cao, S. RSC Adv. 2015, 5, 34498.
(e) Xiong, Y.; Huang, T.; Ji, X.; Wu, J.; Cao, S. Org. Biomol. Chem. 2015, 13, 7398.
(f) Dai, W.; Shi, H.; Zhao, X.; Cao, S. Org. Lett. 2016, 18, 4284.
(g) Kong, L.; Zhou, X.; Li, X. Org. Lett. 2016, 18, 6320.
[6] (a) Liu, G. Org. Biomol. Chem. 2012, 10, 6243.
(b) Akana, J. A.; Bhattacharyya, K. X.; Müller, P.; Sadighi, J. P. J. Am. Chem. Soc. 2007, 129, 7736.
(c) Gorske, B. C.; Mbofana, C. T.; Miller, S. J. Org. Lett. 2009, 11, 4318.
(d) Schuler, M.; Silva, F.; Bobbio, C.; Tessier, A.; Gouverneur, V. Angew. Chem. Int. Ed. 2008, 47, 7927.
(e) de Haro, T.; Nevado, C. Chem. Commun. 2011, 47, 248;
(f) Lan, Y.; Hammond, G. B. Org. Lett. 2002, 4, 2437.
(g) Zhou, C.; Ma, Z.; Gu, Z.; Fu, C.; Ma, S. J. Org. Chem. 2008, 73, 772.
(h) Cui, H. F.; Chai, Z.; Zhao, G.; Zhu, S. Z. Chin. J. Chem. 2009, 27, 189.
(i) Lü, B.; Fu, C.; Ma, S. Org. Biomol. Chem. 2010, 8, 274.
[7] (a) Couve-Bonnaire, S.; Cahard, D.; Pannecoucke, X. Org. Biomol. Chem. 2007, 5, 1151.
(b) Yanai, H.; Taguchi, T. Eur. J. Org. Chem. 2011, 5939.
(c) Landelle, G.; Bergeron, M.; Turcotte-Savard, M.-O.; Paquin, J.-F. Chem. Soc. Rev. 2011, 40, 2867.
(d) Champagne, P. A.; Desroches, J.; Hamel, J.-D.; Vandamme, M.; Paquin, J.-F. Chem. Rev. 2015, 115, 9073.
(e) Drouin, M.; Hamel, J.-D.; Paquin, J.-F. Synlett 2016, 27, 821.
(f) Zhang, X.; Cao, S. Tetrahedron Lett. 2017, 58, 375.
[8] Schlosser, M.; Zimmermann, M. Synthesis 1969, 75.
[9] Cox, D. G.; Gurusamy, N.; Burton, D. J. J. Am. Chem. Soc. 1985, 107, 2811.
[10] (a) Lei, X. S.; Dutheuil, G.; Pannecoucke, X.; Quirion, J. C. Org. Lett. 2004, 6, 2101.
(b) Zoute, L.; Dutheuil, G.; Quirion, J.-C.; Jubault, P.; Pannecoucke, X. Synthesis 2006, 3409.
[11] Burton, D. J.; Yang, Z.-Y.; Qiu, W. Chem. Rev. 1996, 96, 1641.
[12] Machleidt, H.; Wessendorf, R. Justus Liebigs Ann. Chem. 1964, 674, 1.
[13] Moghadam, G. E.; Penne, J. S. Bull. Soc. Chim. Fr. 1985, 448.
[14] Sano, S.; Matsumoto, T.; Nakao, M. Tetrahedron Lett. 2014, 55, 4480.
[15] Chevrie, D.; Lequeux, T.; Demoute, J. P.; Pazenok, S. Tetrahedron Lett. 2003, 44, 8127.
[16] Ghosh, A. K.; Zajc, B. Org. Lett. 2006, 8, 1553.
[17] (a) Zajc, B.; Kake, S. Org. Lett. 2006, 8, 4457.
(b) Pfund, E.; Lebargy, C.; Rouden, J.; Lequeux, T. J. Org. Chem. 2007, 72, 7871.
[18] (a) Ghosh, A. K.; Banerjee, S.; Sinha, S.; Kang, S. B.; Zajc, B. J. Org. Chem. 2009, 74, 3689.
(b) He, M.; Ghosh, A. K.; Zajc, B. Synlett 2008, 999.
[19] Alonso, D. A.; Fuensanta, M.; Gómez-Bengoa, E.; Nájera, C. Adv. Synth. Catal. 2008, 350, 1823.
[20] Larnaud, F.; Pfund, E.; Linclau, B.; Lequeux, T. Tetrahedron 2014, 70, 5632.
[21] Zhao, Y.; Jiang, F.; Hu, J. J. Am. Chem. Soc. 2015, 137, 5199.
[22] Satoh, T.; Itoh, N.; Onda, K.; Kitoh, Y.; Yamakawa, K. Bull. Chem. Soc. Jpn. 1992, 65, 2800.
[23] Chevrie, D.; Lequeux, T.; Pommelet, J.-C. Org. Lett. 1999, 1, 1539.
[24] Boys, M. L.; Collington, E. W.; Finch, H.; Swanson, S.; Whitehead, J. F. Tetrahedron Lett. 1988, 29, 3365.
[25] (a) Zhang, W.; Huang, W.; Hu, J. Angew. Chem. Int. Ed. 2009, 48, 9858.
(b) Shen, X.; Hu, J. Eur. J. Org. Chem. 2014, 4437.
(c) Liu, Q.; Shen, X.; Ni, C.; Hu, J. Angew. Chem. Int. Ed. 2017, 56, 619.
[26] Welch, J. T.; Herbert, R. W. J. Org. Chem. 1990, 55, 4782.
[27] [Michida, M.; Mukaiyama, T. Chem. Lett. 2008, 37, 890.
[28] (a) Barma, D. K.; Kundeu, A.; Zhang, H.; Mioskowski, C.; Falck, J. R. J. Am. Chem. Soc. 2003, 125, 3218.
(b) Falck, J. R.; Bojet, R.; Barma, D. K.; Bandyopadhyay, A.; Joseph, S.; Mioskowski, C. J. Org. Chem. 2006, 71, 8178.
(c) Feng, Z.; Min, Q.-Q.; Zhao, H.-Y.; Gu, J.-W.; Zhang, X.Angew. Chem. Int. Ed. 2015, 54, 1270.
[29] Lemonnier, G.; Zoute, L.; Dupas, G.; Quirion, J.-C.; Jubault, P. J. Org. Chem. 2009, 74, 4124.
[30] Augustine, J.-K.; Bombrun, A.; Venkatachaliah, S.; Jothi, A. Org. Biomol. Chem. 2013, 11, 8065.
[31] Cao, C.-R.; Ou, S.; Jiang, M.; Liu, J.-T. Org. Biomol. Chem. 2014, 12, 467.
[32] (a) Grundmann, C. Justus. Liebigs Ann. Chem. 1938, 536, 29.
(b) Font, J.; Serratosa, F.; Valls, J. Chem. Commun. 1970, 721.
(c) Kulkowit, S.; McKervey, M. A. J. Chem. Soc., Chem. Commun. 1978, 1069.
(d) Baratta, W.; Del Zotto, A.; Rigo, P. Chem. Commun. 1997, 2163.
(e) Del Zotto, A.; Baratta, W.; Verardo, G.; Rigo, P. Eur. J. Org. Chem. 2000, 2795.
(f) Doyle, M. P.; Hu, W.; Phillips, I. M. Org. Lett. 2000, 2, 1777.
(g) Greenman, K. L.; Carter, D. S.; Van Vranken, D. L. Tetrahedron 2001, 57, 5219.
(h) Doyle, M. P.; Yan, M. J. Org. Chem. 2002, 67, 602;
(i) Li, G.-Y.; Che, C.-M. Org. Lett. 2004, 6, 1621.
(j) Hodgson, D. M.; Angrish, D. Chem. Commun. 2005, 4902.
(k) Chen, S.; Wang, J. Chem. Commun. 2008, 4198;
(l) Xiao, Q.; Ma, J.; Yang, Y.; Zhang, Y.; Wang, J. Org. Lett. 2009, 11, 4732.
(m) Hansen, J. H.; Parr, B. T.; Pelphrey, P.; Jin, Q.; Autschbach, J.; Davies, H. M. L.Angew. Chem. Int. Ed. 2011, 50, 2544.
(n) Xia, Y.; Liu, Z.; Xiao, Q.; Qu, P.; Ge, R.; Zhang, Y.; Wang, J. Angew. Chem. Int. Ed. 2012, 51, 5714.
(o) Hu, M.; He, Z.; Gao, B.; Li, L.; Ni, C.; Hu, J. J. Am. Chem. Soc. 2013, 135, 17302.
(p) Xiao, Q.; Zhang, Y.; Wang, J. Acc. Chem. Res. 2013, 46, 236.
(q) Zhang, D.; Xu, G.; Ding, D.; Zhu, C.; Li, J.; Sun, J. Angew. Chem. Int. Ed. 2014, 53, 11070.
(r) Hu, M.; Ni, C.; Li, L.; Han, Y.; Hu, J. J. Am. Chem. Soc. 2015, 137, 14496.
(s) Zhu, C.; Xu, G.; Liu, K.; Qiu, L.; Peng, S.; Sun, J. Chem. Commun. 2015, 51, 12768.
(t) Zhu, C.; Xu, G.; Ding, D.; Qiu, L.; Sun, J. Org. Lett. 2015, 17, 4244.
(u) Zhang, Z.; Yu, W.; Wu, C.; Wang, C.; Zhang, Y.; Wang, J. Angew. Chem. Int. Ed. 2016, 55, 273.
(v) Feng, S.; Mo, F.; Xia, Y.; Liu, Z.; Liu, Z.; Zhang, Y.; Wang, J. Angew. Chem. Int. Ed. 2016, 55, 15401.
[33] (a) Fuchigami, T.; Hayashi, T.; Konno, A. Tetrahedron Lett. 1992, 33, 3161.
(b) Usuki, Y.; Iwaoka, M.; Tomoda, S. J. Chem. Soc., Chem. Commun. 1992, 1148.
[34] (a) Ivanova, M. V.; Bayle, A.; Besset, T.; Pannecoucke, X.; Poisson, T. Angew. Chem. Int. Ed. 2016, 55, 14141.
(b) Feng, Z.; Min, Q.-Q.; Xiao, Y.-L.; Zhang, B.; Zhang, X.Angew. Chem. Int. Ed. 2014, 53, 1669.
[35] (a) Cao, Z.-Y.; Wang, X.; Tan, C.; Zhao, X.-L.; Zhou, J.; Ding, K. J. Am. Chem. Soc. 2013, 135, 8197.
(b) Cao, Z.-Y.; Zhao, Y.-L.; Zhou, J. Chem. Commun. 2016, 52, 2537.
(c) Zhao, Y.-L.; Cao, Z.-Y.; Zeng, X.-P.; Shi, J.-M.; Yu, Y.-H.; Zhou, J. Chem. Commun. 2016, 52, 3943.
[36] For a review, see:
(a) Decostanzi, M.; Campagne, J.-M.; Leclerc, E. Org. Biomol. Chem. 2015, 13, 7351. For examples, see:
(b) Amii, H.; Kobayashi, T.; Hatamoto, Y.; Uneyama, K. Chem. Commun. 1999, 1323.
(c) Chu, L.; Zhang, X.; Qing, F.-L. Org. Lett. 2009, 11, 2197.
(d) Yuan, Z.; Wei, Y.; Shi, M. Chin. J. Chem. 2010, 28, 1709.
(e) Kashikura, W.; Mori, K.; Akiyama, T. Org. Lett. 2011, 13, 1860. For our continuous efforts in the functionalization of fluorinated enol silyl ethers, see:
(f) Liu, Y.-L.; Zhou, J. Chem. Commun. 2012, 48, 1919.
(g) Liu, Y.-L.; Zeng, X.-P.; Zhou, J. Acta Chim. Sinica. 2012, 70, 1451.
(h) Liu, Y.-L.; Liao, F.-M.; Niu, Y.-F.; Zhao, X.-L.; Zhou, J. Org. Chem. Front. 2014, 1, 742.
(i) Yu, J.-S.; Liu, Y.-L.; Tang, J.; Wang, X.; Zhou, J. Angew. Chem. Int. Ed. 2014, 53, 9512.
(j) Liao, F.-M.; Liu, Y.-L.; Yu, J.-S.; Zhou, F.; Zhou, J. Org. Biomol. Chem. 2015, 13, 8906.
(k) Yu, J.-S.; Zhou, J. Org. Biomol. Chem. 2015, 13, 10968.
(l) Yu, J.-S.; Liao, F.-M.; Gao, W.-M.; Liao, K.; Zuo, R.-L.; Zhou, J. Angew. Chem. Int. Ed. 2015, 54, 7381.
(m) Yu, J.-S.; Zhou, J. Org. Chem. Front. 2016, 3, 298.
(n) Zeng, X.-P.; Zhou, J. J. Am. Chem. Soc. 2016, 138, 8730.
[37] Liao, F.-M.; Cao, Z.-Y.; Yu, J.-S.; Zhou, J. Angew. Chem. Int. Ed. 2017, 56, 2459.
[38] (a) Okoromoba, O. E.; Han, J.; Hammond, G. B.; Xu, B. J. Am. Chem. Soc. 2014, 136, 14381.
(b) Nahra, F.; Patrick, S. R.; Bello, D.; Brill, M.; Obled, A.; Cordes, D. B.; Slawin, A. M. Z.; O'Hagan, D.; Nolan, S. P. ChemCatChem 2015, 7, 240.
(c) Li, Y.; Liu, X.; Ma, D.; Liu, B.; Jiang, H. Adv. Synth. Catal. 2012, 354, 2683.
[39] (a) Lee, S. H.; Schwartz, J. J. Am. Chem. Soc. 1986, 108, 2445.
(b) Tius, M. A.; Kawakami, J. K. Tetrahedron 1995, 51, 3997.
(c) Sommer, H.; Fürstner A. Chem. Eur. J. 2017, 23, 558.
(d) Petasis, N. A.; Yudin, A. K.; Zavialov, I. A.; Prakash, G. K. S.; Olah, G. A. Synlett 1997, 606.
(e) Furuya, T.; Ritter, T. Org. Lett. 2009, 11, 2860.
(f) Tredwell, M.; Gourverneur, V. Org. Biomol. Chem. 2006, 4, 26.
(g) Yang, M.-H.; Matikonda, S. S.; Altman, R. A. Org. Lett. 2013, 15, 3894.
(h) Ye, Y.; Takada, T.; Buchwald, S. L. Angew. Chem. Int. Ed. 2016, 55, 15559.
[40] (a) Qiu, S.; Xu, T.; Zhou, J.; Guo, Y.; Liu, G. J. Am. Chem. Soc. 2010, 132, 2856;
(b) Shao, Q.; Huang, Y. Chem. Commun. 2015, 51, 6584.
/
| 〈 |
|
〉 |