

Chinese Journal of Organic Chemistry >
Research Progress for the Thiolysis Reaction of Halobenzonitrile
Received date: 2017-07-03
Revised date: 2017-09-23
Online published: 2017-10-20
Supported by
Project supported by the National Natural Science Foundation of China (No. 21362019), the Natural Science Foundation of Inner Mongolia (No. 2016MS0207) and the "Light of West China" Program of Chinese Academy of Sciences.
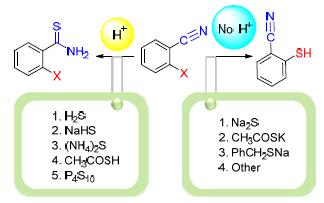
Halobenzonitrile is an important raw material in the fields of medicine, pesticide and materials. Halobenzonitrile has a nitrile group and a halo group which all could react with nucleophile reagents. The law for the selective synthesis of halothiobenzamide or mercaptobenzonitrile from the reaction of halobenzonitrile and different sulfur source is summarized. It is found that the halothiobenzamide product could be synthesized under protonic acid condition from halobenzonitrile, while the mercaptobenzonitrile product would be formed under the alkaline conditions.
Li Shanshan , Hong Hailong , Han Limin , Zhang Tianmiao , Wang Yunlong , Zhu Ning . Research Progress for the Thiolysis Reaction of Halobenzonitrile[J]. Chinese Journal of Organic Chemistry, 2018 , 38(2) : 304 -315 . DOI: 10.6023/cjoc201707002
[1] (a) Garcia Alejandre, J. J.; Arevalo Salas, A. R.; Reyes Rios, G.; Crestani Gutierrez, M. G.; Barrios Francisco, R. Chem. Commun. 2011, 47, 10121.
(b) Feng, W.; Dong, H.; Niu, L.; En, X.; Huo, L.; Bai, G. J. Mater. Chem. A 2015, 3, 19807.
(c) Adam, R.; Alberico, E.; Baumann, W.; Drexler, H. -J.; Jackstell, R.; Junge, H.; Beller, M. Chem. -Eur. J. 2016, 22, 4991.
[2] (a) Theodorou, V.; Paraskevopoulos, G.; Skobridis, K. ARKIVOC 2015, 101.
(b) Marce, P.; Lynch, J.; Blacker, A. J.; Williams, J. M. J. Chem. Commun. 2016, 52, 1436.
[3] Louvel, J.; Guo, D.; Agliardi, M.; Mocking, T. A. M.; Kars, R.; Pham, T. P.; Xia, L.; de Vries, H.; Brussee, J.; Heitman, L. H.; Ijzerman, A. P. J. Med. Chem. 2014, 57, 3213.
[4] Hua, G.; Du, J.; Slawin, A. M. Z.; Woollins, J. D. Synlett 2014, 25, 2189.
[5] (a) Ichinokawa, N.; Onozaki, Y. WO 2016143655, 2016[Chem. Abstr. 2016, 165, 365462].
(b) Flesch, D.; Gabler, M.; Lill, A.; Gomez, R. C.; Steri, R.; Schneider, G.; Stark, H.; Schubert-Zsilavecz, M.; Merk, D. Bioorg. Med. Chem. Lett. 2015, 23, 3490.
[6] (a) Ghodsinia, S. S. E.; Akhlaghinia, B. RSC Adv. 2015, 5, 49849.
(b) Esmaeilpour, M.; Javidi, J.; Zahmatkesh, S. Appl. Organomet. Chem. 2016, 30, 897.
[7] Garcia, J. J.; Zerecero-Silva, P.; Reyes-Rios, G.; Crestani, M. G.; Arevalo, A.; Barrios-Francisco, R. Chem. Commun. 2011, 47, 10121.
[8] Tanaka, H.; Shizu, K.; Nakanotani, H.; Adachi, C. J. Phys. Chem. C 2014, 118, 15985.
[9] Ding, G.; Han, H.; Jiang, T.; Wu, T.; Han, B. Chem. Commun. 2014, 50, 9072.
[10] Nikoorazm, M.; Ghorbani-Choghamarani, A.; Noori, N.; Tahmasbi, B. Appl. Organomet. Chem. 2016, 30, 843.
[11] Paik, S.; Jung, M. G. Bull. Korean Chem. Soc. 2012, 33, 689.
[12] Rostami, A.; Rostami, A.; Iranpoor, N.; Zolfigol, M. A. Tetrahedron Lett. 2016, 57, 192.
[13] Kassaee, M. Z.; Motamedi, E.; Movassagh, B.; Poursadeghi, S. Synthesis 2013, 45, 2337.
[14] Zhang, S.; Karra, K.; Koe, A.; Jin, J. Tetrahedron Lett. 2013, 54, 2452.
[15] Karimi, B.; Vafaeezadeh, M.; Akhavan, P. F. ChemCatChem 2015, 7, 2248.
[16] (a) Vo, N. H.; Chen, S.; Che, Q.; Xie, Y. WO 2007087427, 2007[Chem. Abstr. 2007, 147, 235154].
(b) Crane, L. J.; Anastassiadou, M.; Stigliani, J. -L.; Baziard-Mouysset, G.; Payard, M. Tetrahedron 2004, 60, 5325.
(c) Zhang, J. CN 102491955, 2012[Chem. Abstr. 2012, 157, 45166].
(d) Taldone, T.; Patel, P. D.; Patel, H. J.; Chiosis, G. Tetrahedron Lett. 2012, 53, 2548.
[17] Shen, G. L.; Xu, T. J. J. Liaoyang Petrochem. College 1998, 14, 1. (in Chinese). (沈国良, 徐铁军, 辽阳石油化专学报, 1998, 14, 1.)
[18] Chen, J. -L. WO 2005086904, 2005[Chem. Abstr. 2005, 143, 326092.]
[19] (a) Liu, H.; Jiang, X. Chem. -Asian J. 2013, 8, 2546.
(b) Wei, J.; Li, Y.; Jiang, X. Org. Lett. 2016, 18, 340.
(c) Tan, W.; Wei, J.; Jiang, X. Org. Lett. 2017, 19, 2166.
(d) Nguyen, T. B. Adv. Synth. Catal. 2017, 10, 1066.
[20] Fairfull, A. E. S.; Lowe, J. L.; Peak, D. A. J. Chem. Soc. 1952, 742.
[21] Bjorklund, M. D. C.; Michael D. J. Heterocycl. Chem. 1980, 17, 819.
[22] Hull, J. W.; Romer, D. R.; Adaway, T. J.; Podhorez, D. E. Org. Process Res. Dev. 2009, 13, 1125.
[23] Cummings, C. G.; Hamilton, A. D. Tetrahedron 2013, 69, 1663.
[24] Bagley, M.; Chapaneri, K.; Glover, C.; Merritt, E. Synlett 2004, 36, 2615.
[25] Khosropour, A. R.; Noei, J.; Mirjafari, A. J. Iran. Chem. Soc. 2010, 7, 752.
[26] Manaka, A.; Sato, M. Synth. Commun. 2005, 35, 761.
[27] Tisdell, F. E.; Johnson, P. L.; Pechacek, J. T.; Suhr, R. G.; Devries, D. H.; Denny, C. P.; Ash, M. L. WO 2000024739, 2000[Chem. Abstr. 2000, 132, 308343].
[28] Nitlikar, L. H.; Sangshetti, J. N.; Shinde, D. B. Anti-Inflammatory Anti-Allergy Agents Med. Chem. 2014, 13, 128.
[29] Chao, E. Y. -H.; Haffner, C. D.; Lambert, M. H.; Maloney, P. R.; Sierra, M. L.; Sternbach, D. D.; Sznaidman, M. L.; Willson, T. M.; Xu, H. E.; Gellibert, F. J. WO 2001000603, 2001[Chem. Abstr. 2001, 134, 86235].
[30] Brown, A. D.; Davis, R. D.; Fitzgerald, R. N.; Glover, B. N.; Harvey, K. A.; Jones, L. A.; Liu, B.; Patterson, D. E.; Sharp, M. J. Org. Process Res. Dev. 2009, 13, 297.
[31] Tisdell, F. E.; Johnson, P. L.; Pechacek, J. T.; Suhr, R. G.; Devries, D. H.; Denny, C. P.; Ash, M. L. WO 2000024739, 2000[Chem. Abstr. 2000, 132, 308343].
[32] Luqman, A.; Blair, V. L.; Brammananth, R.; Crellin, P. K.; Coppel, R. L.; Andrews P. C. Eur. J. Inorg. Chem. 2015, 4935.
[33] Oschatz, S.; Brunzel, T.; Wu, X. F.; Langer, P. Org. Biomol. Chem. 2015, 13, 1150.
[34] Mahammed, K. A.; Jayashankara, V. P.; Rai, N. P.; Raju K. M.; Arunachalam, P. N. Synlett 2009, 2338.
[35] Darabi, H. R.; Roozkhosh, A.; Aghapoor, K. Aust. J. Chem. 2016, 69, 198.
[36] Gauthier, J. Y.; Lebel, H. Phosphorus, Sulfur Silicon Relat. Elem. 1994, 95, 325.
[37] Lowe, A.; Whittaker, M.; Dieterich, P.; Polywka, M. E. C. WO 2005095425, 2005[Chem. Abstr. 2005, 143, 378578].
[38] Zhou, Z.; Li, Z.; Wang, Q.; Liu, B.; Li, K.; Zhao, G.; Zhou, Q.; Tang, C. J. Organomet. Chem. 2006, 691, 5790.
[39] Van Zyl, W. E.; Fackler, J. P. Phosphorus, Sulfur Silicon Relat. Elem. 2000, 167, 117.
[40] Kaboudin, B.; Elhamifar, D. Synthesis 2006, 224.
[41] Kaboudin, B.; Elhamifar, D.; Farjadian, F. Org. Prep. Proced. Int. 2006, 38, 412.
[42] Kaboudin, B.; Norouzi, H. Synthesis 2004, 2035.
[43] Martelli, A.; Testai, L.; Citi, V.; Marino, A.; Pugliesi, I.; Barresi, E.; Nesi, G.; Rapposelli, S.; Taliani, S.; Da Settimo, F.; Breschi, M. C.; Calderone, V. ACS Med. Chem. Lett. 2013, 4, 904.
[44] Shabana, R.; Meyer, H. J.; Lawesson, S. O. Phosphorus, Sulfur Silicon Relat. Elem. 1985, 25, 297.
[45] Pudovik, A. N.; Cherkasov, R. A.; Zimin, M. G.; Zabirov, N. G. Russ. Chem. Bull. 1979, 28, 805.
[46] Soh, C. H.; Chui, W. K.; Lam, Y. J. Comb. Chem. 2006, 8, 464.
[47] Benner, S. A. Tetrahedron Lett. 1981, 22, 1851.
[48] Cherkasov, R. A.; Kutyrev, G. A.; Pudovik, A. N. Tetrahedron 1985, 41, 2567.
[49] Nagl, M.; Panuschka, C.; Barta, A.; Schmid, W. Synthesis 2008, 4012.
[50] Dixon, D. D.; Grina, J.; Josey, J. A.; Rizzi, J. P.; Schlachter, S. T.; Wallace, E. M.; Wang, B.; Wehn, P.; Xu, R.; Yang, H. WO 2015035223, 2015[Chem. Abstr. 2015, 162, 413231].
[51] Cerdeira, A. C.; Simão, D.; Santos, I. C.; Machado, A.; Pereira, L. C. J.; Waerenborgh, J. C.; Henriques, R. T.; Almeida, M. Inorg. Chim. Acta 2008, 361, 3836.
[52] Evans, T. L.; Kinnard, R. D. J. Org. Chem. 1983, 48, 2496.
[53] Nie, W. M. S. Thesis, Dalian University of Technology, Dalian, 2011(in Chinese). (聂薇, 硕士论文, 大连理工大学, 大连, 2011.)
[54] Zhang, S. M. WO 2013017026, 2013[Chem. Abstr. 2013, 158, 331037].
[55] Crich, D.; Sharma, I. Angew. Chem., Int. Ed. 2009, 48, 7591.
[56] Kalugin, V. E.; Shestopalov, A. M. Russ. Chem. Bull. 2014, 63,
[57] Qiao, S.; Xie, K.; Qi, J. Chin. J. Chem. 2010, 28, 1441.
[58] Tickner, A. M.; Huang, G. K.; Gombatz, K.; Mills, R. J.; Novack, V.; Webb, K. S. Synth. Commun. 1995, 25, 2497.
[59] Dai, M. CN 103130738, 2013[Chem. Abstr. 2013, 159, 91976].
[60] Korn, T. J.; Knochel, P. Synlett 2005, 1185.
[61] Liu, Y.; Kim, J.; Seo, H.; Park, S.; Chae, J. Adv. Synth. Catal. 2015, 357, 2205.
[62] Takikawa, Y.; Takizawa, S. Nippon Kagaku Kaishi 1972, 766.
/
| 〈 |
|
〉 |