

Chinese Journal of Organic Chemistry >
Recent Advances of α-Aryl Vinyl Azides in Nitrogen Heterocycle Synthesis
Received date: 2017-09-15
Revised date: 2017-10-13
Online published: 2017-11-15
Supported by
Project supported by the Natural Science Foundation of Jiangsu Province (No. BK20130748).
Nitrogen heterocyclic compounds can be found in various natural products, pharmaceutical chemistry and material chemistry. Due to azide group linked to olefins, α-aryl vinyl azide has unique properties, which can act as electrophilic reagents, nucleophilic reagent, or radical acceptor. Diverse reaction pathways of α-aryl vinyl azide provide great opportunities to generate highly reactive intermediates with unusual or unconventional reactivities, making it possible to develop novel reaction. Recently, more and more synthetic chemists used α-aryl vinyl azide as a key three atoms synthon for the construction of diverse structurally complex N-heterocyclic compounds. This review will introduce systematically the reactivities of α-aryl vinyl azide and the developments of the recent application of α-aryl vinyl azide in nitrogen heterocycle synthesis, including mechanism, reaction characteristics and application study, thus it may be helpful for the research on nitrogen heterocycle synthesis.
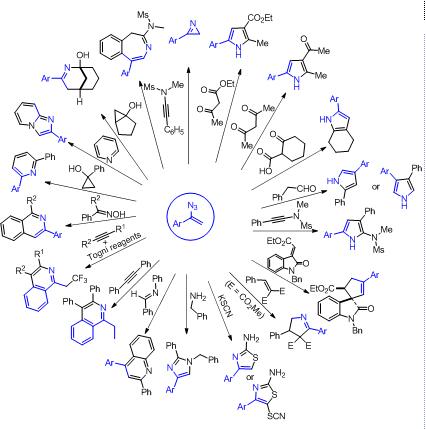
Key words: α-aryl vinyl azide; synthon; nitrogen heterocyclic compounds; application
Yan Jun , Ji Xiaoyue , Hua Shugui , Wang Jing . Recent Advances of α-Aryl Vinyl Azides in Nitrogen Heterocycle Synthesis[J]. Chinese Journal of Organic Chemistry, 2018 , 38(4) : 791 -801 . DOI: 10.6023/cjoc201709025
[1] For selected reviews, see:(a) Carey, J. S.; Laffan, D.; Thomson, C.; Williams, M. T. Org. Biomol. Chem. 2006, 4, 2337.
(b) Welsch, M. E.; Snyder, S. A.; Stockwell, B. R. Curr. Opin. Chem. Biol. 2010, 14, 347.
(c) Dandapani, S.; Marcaurelle, L. A. Curr. Opin. Chem. Biol. 2010, 14, 362.
(d) Tohme, R.; Darwiche, N.; Gali-Muhtasib, H. Molecules 2011, 16, 9665.
(e) Thomas, G. L.; Johannes, C. W. Curr. Opin. Chem. Biol. 2011, 15, 516.
(f) Zhang, Z.; Zheng, X.; Guo, C. Chin. J. Org. Chem. 2016, 36, 1241.
(g) Zhang, J.; Liu, J.; Ma, Y.; Cheng, P. Chin. J. Org. Chem. 2017, 37, 555.
[2] For recent reviews, see:(a) Gribble, G. W.; Joule, J. A. Progress in Heterocyclic Chemistry, Vol. 20, Elsevier, Oxford, 2008, and others in this series.
(b) Katritzky, A. R.; Ramsden, C. A.; Scriven, E. F. V.; Taylor, R. J. K. Comprehensive Heterocyclic Chemistry Ⅲ, Pergamon, Oxford, 2008.
(c) Katritzky, A. R.; Rees, C. W.; Scriven, E. F. V.; McKillop, A. Comprehensive Heterocyclic Chemistry Ⅱ, Pergamon, Oxford, 1996and references therein.
[3] (a) Hassner, A.; Fowler, F. W. J. Org. Chem. 1968, 33, 2686.
(b) Brase, S.; Gil, C.; Knepper, K.; Zimmermann, V. Angew. Chem., Int. Ed. 2005, 44, 5188.
(c) Gu, P.; Su, Y.; Wu, X. P.; Sun, J.; Liu, W.; Xue, P.; Li, R. Org. Lett. 2012, 14, 2246.
[4] (a) Nair, V.; Tesmol, G. G. Tetrahedron Lett. 2000, 41, 3199.
(b) Zhu, W.; Ma, D. Chem. Commun. 2004, 888.
(c) Telvekar, V. N.; Takale, B. S.; Bachhav, H. M. Tetrahedron Lett. 2009, 50, 5056.
(d) Kupracz, L.; Hartwig, J.; Wegner, J.; Ceylan, S.; Kirschning, A. Beilstein J. Org. Chem. 2011, 7, 1441.
(e) Li, X.; Liao, S.; Wang, Z.; Zhang, L. Org. Lett. 2017, 19, 3687.
[5] Griess, P. Liebigs Ann. Chem. 1866, 137, 39.
[6] For recent reviews on organic azides, see:(a) Brase, S.; Gil, C.; Knepper, K.; Zimmermann, V. Angew. Chem., Int. Ed. 2005, 44, 5188.
(b) Moses, J. E.; Moorhouse, A. D. Chem. Soc. Rev. 2007, 36, 1249.
(c) Driver, T. G. Org. Biomol. Chem. 2010, 8, 3831.
[7] Select examples:(a) Chiba, S.; Wang, Y.-F.; Lapointe, G.; Narasaka, K. Org. Lett. 2008, 10, 313.
(b) Wang, Y.-F.; Toh, K. K.; Chiba, S.; Narasaka, K. Org. Lett. 2008, 10, 5019.
(c) Wang, Y.-F.; Chiba, S. J. Am. Chem. Soc. 2009, 131, 12570.
(d) Wang, Y.-F.; Toh, K. K.; Ng, E. P. J.; Chiba, S. J. Am. Chem. Soc. 2011, 133, 6411.
(e) Wang, Y.-F.; Toh, K. K.; Lee, J.-Y.; Chiba, S. Angew. Chem., Int. Ed. 2011, 50, 5927.
(f) Chen, F.; Shen, T.; Cui, Y.; Jiao, N. Org. Lett. 2012, 14, 4926.
(g) Wang, Y.-F.; Lonca, G. H.; Runigo, M. L.; Chiba, S. Org. Lett. 2014, 16, 4272.
[8] For some recent reviews, see:(a) Murphee, S. S.; Padwa, A. In Progress Heterocyclic Chemistry, Vol. 9, Eds.:Scriven, E. F. V.; Suschitzky, H., Pergamon Press, Oxford, 1997.
(b) Pearson, W. H.; Lian B. W.; Bergmeier, S. C. In Comprehensive Heterocyclic Chemistry Ⅱ, Vol. 1A, Eds.:Katritzky, A. R.; Rees, C. W.; Scriven, E. F. V., Pergamon Press, Oxford, 1996, Chapter 1.
(c) Heimgartner, H. Angew. Chem., Int. Ed. 1991, 30, 238.
[9] (a) Ray, C. A.; Risberg, E.; Somfai, P. Tetrahedron. 2002, 58, 5983.
(b) Palacios, F.; de Retana, A. M. O.; de Marigorta, E. M.; de los Santos, J. M. Org. Prep. Proced. Int. 2002, 34, 219.
(c) Timen, A. S.; Fischer, A.; Somfai, P. Chem. Commun. 2003, 34, 1150.
[10] For recent reviews on 2H-azirines, see:(a) Palacios, F.; Retana, A. M. D.; Marigorta, E. M. D.; Santos, J. D. L. Eur. J. Org. Chem. 2001, 2401.
(b) Palacios, F.; Retana, A. M. O. D.; Marigorta, E. M. D.; Santos J. M. D. L. Org. Prep. Proced. Int. 2002, 34, 219.
(c) Khlebnikov, A. F.; Novikov, M. S. Tetrahedron 2013, 69, 3363.
[11] (a) Hortmann, A. G.; Robertson, D. A.; Gillard, B. K. J. Org. Chem. 1972, 37, 322.
(b) Hassner, A.; Fowler, F. W. J. Am. Chem. Soc. 1968, 90, 2869.
(c) Pinho e Melo, T. M. V. D.; Lopes, C. S. J.; Cardoso, A. L.; Rocha Gonsalves, A. M.d'A. Tetrahedron 2001, 57, 6203.
[12] Asa Sjöholm Timen, Risberg E, Somfai P. ChemInform 2003, 44, 5339.
[13] Singh, P. N. D.; Carter, C. L.; Gudmundsdottir, A. D. Tetrahedron Lett. 2003, 44, 6763.
[14] (a) Muchowski, J. M. Adv. Med. Chem. 1992, 1, 109.
(b) Cozzi, P.; Mongelli, N. Curr. Pharm. Des. 1998, 4, 181.
(c) Fürstner, A. Angew. Chem., Int. Ed. 2003, 42, 3582.
(d) Balme, G. Angew. Chem., Int. Ed. 2004, 43, 6238.
(e) Andreani, A.; Cavalli, A.; Granaiola, M.; Guardigli, M.; Leoni, A.; Locatelli, A.; Morigi, R.; Rambaldi, M.; Recanatini, M.; Roda, A. J. Med. Chem. 2001, 44, 4011.
(f) Baraldi, P. G.; Nunez, M. C.; Tabrizi, M. A.; De Clercq, E.; Balzarini, J.; Bermejo, J.; Esterez, F.; Romagnodi, R. J. Med. Chem. 2004, 47, 2877.
(g) Srivastava, S. K.; Shefali Miller, C. N.; Aceto, M. D.; Traynor, J. R.; Lewis, J. W.; Husbands, S. M.; J. Med. Chem. 2004, 47, 6645.
[15] Walsh, C. T.; Garneau-Tsodikova, S.; Howard-Jones, A. R. Nat. Prod. Rep. 2006, 23, 517.
[16] (a) Novak, P.; Müller, K.; Santhanam, K. S. V.; Haas, O. Chem. Rev. 1997, 97, 207.
(b) Gale, P. A.; Acc. Chem. Res. 2006, 39, 465.
[17] For selected examples in recent years, see:(a) Wan, X.; Xing, D.; Fang, Z.; Li, B.; Zhao, F.; Zhang, K.; Yang, L.; Shi, Z. J. Am. Chem. Soc. 2006, 128, 12046.
(b) Seregin, I. V.; Gevorgyan, V. J. Am. Chem. Soc. 2006, 128, 12050.
(c) Martln, R.; Rivero, M. R.; Buchwald, S. L. Angew. Chem., Int. Ed. 2006, 45, 7079.
(d) Binder, J. T.; Kirsch, S. F. Org. Lett. 2006, 8, 2151.
(e) Su, S.; Porco, J. A., Jr. J. Am. Chem. Soc. 2007, 129, 7744.
(f) Shindo, M.; Yoshimura, Y.; Hayashi, M.; Soejima, H.; Yoshikawa, T.; Matsumoto, K.; Shishido, K. Org. Lett. 2007, 9, 1963.
(g) St. Cyr, D. J.; Arndtsen, B. A. J. Am. Chem. Soc. 2007, 129, 12366.
(h) Lu, Y.; Arndtsen, B. A. Angew. Chem., Int. Ed. 2008, 47, 5430.
[18] Wang Y. F.; Toh K. K.; Chiba S.; Narasaka, K. Org. Lett. 2008, 10, 5019.
[19] Richert, S. A.; Tsang, P. K. S.; Sawyer, D. T. Inorg. Chem. 1988, 27, 1814.
[20] Chen, F.; Shen, T.; Cui, Y.; Jiao, N. Org. Lett. 2012, 14, 4926.
[21] Pawar, S. K.; Sahani, R. L.; Liu, R. S. Chem.-Eur. J. 2015, 21, 10843.
[22] Zhu, X.; Chiba, S. Chem. Commun. 2016, 52, 2473.
[23] Wu, X. J.; Kassie, F.; Mersch-Sundermann, V. Mutat. Res. 2005, 589, 81.
[24] Chen, B.; Guo, S.; Guo, X.; Zhang, G.; Yu, Y. Org. Lett. 2015, 17, 4698.
[25] (a) Zablotskaya, A.; Segal, I.; Germane, S.; Shestakova, I.; Domracheva, I.; Nesterova, A.; Geronikaki, A.; Lukevies, E. Chem. Heterocycl. Compd. 2002, 38, 859.
(b) Franklin, P. X.; Pillai, A. D.; Rathod, P. D.; Yerande, S. G.; Nivsarkar, M.; Padh, H.; Sudarsanam, V.; Vasu, K. K. Eur. J. Med. Chem. 2008, 43, 129.
(c) Liu, R.; Huang, Z.; Murray, M. G.; Guo, X.; Liu, G. J. Med. Chem. 2011, 54, 5747.
(d) Annadurai, S.; Martinez, R.; Canney, D. J.; Eidem, T.; Dunman, P. M.; Abou-Gharbia, M. Bioorg. Med. Chem. Lett. 2012, 22, 7719.
(e) Smith, B.; Chang, H.-H.; Medda, F.; Gokhale, V.; Dietrich, J.; Davis, A.; Meuillet, E.; Hulme, C. Bioorg. Med. Chem. Lett. 2012, 22, 3567.
[26] (a) Zhong, J. Nat. Prod. Rep. 2009, 26, 382.
(b) Forte, B.; Malgesini, B.; Piutti, C.; Quartieri, F.; Scolaro, A.; Papeo, G. Mar. Drugs 2009, 7, 705.
(c) Midoux, P.; Pichon, C.; Yaouanc, J.-J.; Jaffres, P. -A. J. Pharmacol. 2009, 157, 166.
(d) Xiong, F.; Chen, X.-X.; Chen, F.-E.; Tetrahedron:Asymmetry 2010, 21, 665.
[27] (a) Lee, R. J. C.; Timmermans, P. C.; Gallaghr, T. F.; Kumar, S.; McNully, D.; Blumenthal, M.; Heys, J. R. Nature 1994, 372, 739.
(b) Antolini, M.; Bozzoli, A.; Ghiron, C.; Kennedy, G.; Rossi, T.; Ursini, A. Bioorg. Med. Chem. Lett. 1999, 9, 1023.
(c) Wang, L.; Woods, K. W.; Li, Q.; Barr, K. J.; McCroskey, R. W.; Hannick, S. M.; Gherke, L.; Credo, R. B.; Hui, Y.-H.; Marsh, K.; Warner, R.; Lee, J. Y.; Zielinsky-Mozng, N.; Frost, D.; Rosenberg, S. H.; Sham, H. L. J. Med. Chem. 2002, 45, 1697.
(d) Dietrich, J.; Gokhale, V.; Wang, X.-D.; Hurley, L. H.; Flynn, G. A. Bioorg. Med. Chem. 2010, 18, 292.
(e) Cho, H.-J.; Gee, H.-G.; Baek,K.-H.; Ko, S.-K.; Park, J.-K.; Lee, H.; Kim, N.-D.; Lee, M.-G.; Shin, I. J. Am. Chem. Soc. 2011, 133, 20267.
[28] (a) Du, H.; He, Y.; Rasapalli, S.; Lovely, C.-J. Synlett 2006, 965.
(b) Bellina, F.; Cauteruccio, S.; Rossi, R. Tetrahedron 2007, 63, 4571.
(c) Kaniyo, S.; Yamamoto, Y. Chem.-Asian J. 2007, 2, 568.
(d) Bellina, F.; Rossi, R. Adv. Synth. Catal., 2010, 352, 1223.
[29] Xiang, L.; Niu, Y.; Pang, X.; Yang, X. D.; Yan, R. L. Chem. Commun. 2015, 6598.
[30] (a) Chen, F.; Shen, T.; Cui, Y.; Jiao, N. Org. Lett. 2012, 14, 4926.
(b) Donthiri,R. R.; Pappula, V.; Reddy, N. N. K.; Bairagi, D.; Adimurthy, S. J. Org. Chem. 2014, 79, 11277.
(c) Li, T.; Xin, X.; Wang, C.; Wang, D.; Wu, F.; Li, X.; Wan, B. Org. Lett. 2014, 16, 4806.
[31] (a) Lan, R.; Liu, Q.; Fan, P.; Lin, S.; Fernando, S. R.; McCallion, D.; Pertwee, R.; Makriyannis, A. J. Med. Chem.; 1999; 42, 769.
(b) Ballesteros, P.; Claramunt, R. M.; Escolastico, C.; Maria, M. D. S.; Elguero, J. J. Org. Chem. 2002,57, 1873.
(c) Barreiro, E. J.; Camara, C. A.; Verli, H.; Brazil-Mas, L.; Castro, N. G.; Cintra, W. M.; Aracava,Y.; Rodrigues, C. R.; Fraga, C. A. M. J. Med. Chem. 2003, 46, 1144.
(d) Christodoulou,M. S.; Liekens, S.; Kasiotis, K. M.; Haroutounian, S. A. Bioorg. Med. Chem. 2010, 18, 4338.
(e) Rashad, A. E.; Hegab, M. I.; Abdel-Megeid, R. E.; Micky, J. A.; Abdel-Megeid, F. M. E. Bioorg. Med. Chem. 2008, 16, 7102.
(f) Fustero, S.; Sanchez-Rosello, M.; Barrio, P.; Simon-Fuentes, A. Chem. Rev. 2011, 111, 6984.
[32] Hu, J.; Cheng, Y.; Yang, Y.; Rao, Y. Chem. Commun. 2011, 47, 10133.
[33] (a) Michael, J. P. Nat. Prod. Rep. 1997, 14, 605.
(b) Xu, M.; Wagerle, T.; Long, J. K.; Lahm, G. P.; Barry, J. D.; Smith, R. M. Bioorg. Med. Chem. Lett. 2014, 24, 4026.
[34] (a) Francio, G.; Faraone, F.; Leitner, W. Angew. Chem., Int. Ed. 2000, 39, 1428.
(b) Rueping, M.; Antonchick, A. P.; Theissmann, T. Angew. Chem., Int. Ed. 2006, 45, 3683.
[35] (a) Bakhshi, A.; Bhalla, G. J. Sci. Ind. Res. 2004, 63, 715.
(b) Kim,J. I.; Shin, I.-S.; Kim, H.; Lee, J.-K. J. Am. Chem. Soc. 2005, 127, 1614.
[36] Zhu, X.; Wang, Y. F.; Zhang, F. L.; Chiba, S. Chem. Asian J. 2014, 9, 2458.
[37] (a) Kletsas, D.; Li, W.; Han, Z.; Papadopoulos, V. Biochem. Pharmacol. 2004, 67, 1927.
(b) Muscarella, D. E.; O'Brian, K. A.; Lemley, A. T.; Bloom, S. E. Toxicol. Sci. 2003, 74, 66.
[38] (a) Sweetman, B. A.; Muller-Bunz, H.; Guiry, P. J. Tetrahedron Lett. 2005, 46, 4643.
(b) Durola, F.; Sauvage, J. P.; Wenger, O. S. Chem. Commun. 2006, 171.
(c) Lim, C. W.; Tissot, O.; Mattison, A.; Hooper, M. W.; Brown, J. M.; Cowley, A. R.; Hulmes, D. I.; Blacker, A. J. Org. Process Res. Dev. 2003, 7, 379.
[39] (a) Fang, K. H.; Wu, L. L.; Huang, Y. T.; Yang, C. H.; Sun, I. W. Inorg. Chim. Acta 2006, 359, 441.
(b) Zhao, Q.; Liu, Q. S.; Shi, M.; Wang, C.; Yu, M.; Li, L.; Li, F.; Yi, T.; Huang, C. Inorg. Chem. 2006, 45, 6152.
(c) Tsuboyama, A.; Iwawaki, H.; Furugori, M.; Mukaide, T.; Kamatani, J.; Igawa, S.; Moriyama, S. T.; Miura, S.; Takiguchi, T.; Okada, S.; Hoshino, M.; Ueno, K. J. Am. Chem. Soc. 2003, 125, 12971.
[40] Wang, Y.-F.; Toh, K. K.; Lee, J.-Y.; Chiba, S. Angew. Chem., Int. Ed. 2011, 50, 5927.
[41] Liu, K.; Chen, S.; Li, X. G.; Liu, P. N. J. Org. Chem. 2015, 81, 265.
[42] Zhu, Z. Z.; Tang, X. D.; Li, X. W.; Wu, W. Q.; Deng, G. H.; Jiang, H. F. J. Org. Chem. 2016, 81, 1401.
[43] (a) Kock, I.; Heber, D.; Weide, M.; Wolschendorf, U.; Clement, B. J. Med. Chem. 2005, 48, 2772.
(b) Bernardo, P. H.; Wan, K.-F.; Sivaraman, T.; Xu, J.; Moore, F. K.; Hung, A. W.; Mok, H. Y. K.; Yu, V. C.; Chai, C. L. L. J. Med. Chem. 2008, 51, 6699.
(c) Cappoen, D.; Jacobs, J.; Van, T. N.; Claessens, S.; Diels, G.; Anthonissen, R.; Einarsdottir, T.; Fauville, M.; Verschaeve, L.; Huygen, K.; Kimpe, N. D. Eur. J. Med. Chem. 2012, 48, 57.
(d) Cappoen, D.; Claes, P.; Jacobs, J.; Anthonissen, R.; Mathys, V.; Verschaeve, L.; Huygen, K.; Kimpe, N. D. J. Med. Chem. 2014, 57, 2895.
(e) Naidua, K. M.; Nagesha, H. N.; Singhb, M.; Sriramc, D.; Yogeeswaric, P.; Sekhara, K. V. G. C. Eur. J. Med. Chem. 2015, 92, 415.
[44] For selected examples, see:(a) Mehta, B. K.; Yanagisawa, K.; Shiro, M.; Kotsuki, H. Org. Lett. 2003, 5, 1605.
(b) Gerfaud, T.; Neuville, L.; Zhu, J. Angew. Chem., Int. Ed. 2009, 48, 572.
(c) Candito, D. A.; Lautens, M. Angew. Chem., Int. Ed. 2009, 48, 6713.
(d) Zhang, L.; Ang, G. Y.; Chiba, S. Org. Lett. 2010, 12, 3682.
(e) Deb, I.; Yoshikai, N. Org. Lett. 2013, 15, 4254.
(f) Tummatorn, J.; Krajangsri, S.; Norseeda, K.; Thongsornkleeb, C.; Ruchirawat, S. Org. Biomol. Chem. 2014, 12, 5077.
(g) Li, J.; Wang, H.; Sun, J.; Yang, Y.; Liu, L. Org. Biomol. Chem. 2014, 12, 7904.
(h) Jiang, H.; An, X.; Tong, K.; Zheng, T.; Zhang, Y.; Yu, S. Angew. Chem., Int. Ed. 2015, 54, 4055.
[45] Yang, J. C.; Zhang, J. J.; Guo, L. N. Org. Biomol. Chem. 2016, 14, 9806.
[46] Wang, Y. F.; Lonca, G. H.; Le, R. M.; Chiba, S. Org. Lett. 2014, 16, 4272.
[47] Mackay, E.; Studer, A. Chem.-Eur. J. 2016, 22, 13455.
[48] Sun, X.; Yu, S. Chem. Commun. 2016, 52, 10898.
[49] Quesne, P. W. L. J. Nat. Prod. 1997, 60, 202.
[50] Wang, Y. F.; Chiba, S. J. Am. Chem. Soc. 2009, 131, 12570.
[51] Donthiri, R. R.; Pappula, V.; Reddy, N. N. K.; Bairagi, D.; Adimurthy, S. J. Org. Chem. 2014, 79, 11277.
[52] (a) Enguehard-Gueiffier, C.; Gueiffier, A. Med. Chem. 2007, 7, 888.
(b) Lhassani, M.; Chavignon, O.; Chezal, J.-M.; Teulade, J.-C.; Chapat, J.-P.; Snoeck, R.; Andrei, G.; Balzarini, J.; De Clercq, E.; Gueiffier, A. Eur. J. Med. Chem. 1999, 34, 271.
(c) Rupert, K. C.; Henry, J. R.; Dodd, J. H.; Wadsworth, S. A.; Cavender, D. E.; Olini, G. C.; Fahmy, B.; Siekierka, J. Bioorg. Med. Chem. Lett. 2003, 13, 347.
[53] For a review on synthesis of 2-azabicyclo[3.3.1] nonanes, see:Bonjoch, J.; Diaba, F.; Bradshaw, B.; Farmacia, F. Synthesis 2011, 993.
/
| 〈 |
|
〉 |