

Chinese Journal of Organic Chemistry >
Recent Advance of Palladium-Catalyzed Cross-Coupling Reactions of Organoalanes with Electrophiles Reagents
Received date: 2017-06-25
Revised date: 2017-10-23
Online published: 2017-11-15
Supported by
Project supported by the Sichuan Provincial Department of Science and Technology (No. 2015NZ0033).
Organoaluminum compounds are excellent nucleophiles for organic reactions because of their high reactivities, the high Lewis acidity of the aluminum center, and their low toxicities. Therefore, organoalanes are widely applied in cross-coupling reactions. In this paper, recent research results about the organoaluminum reagents applied in cross-coupling reactions catalyzed by palladium are reviewed, involving various reaction systems.
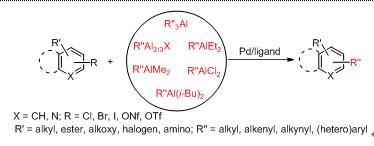
Key words: palladium; organoalanes; cross-coupling reaction; catalyze
Li Qinghan , Shao Xuebei , Zhang Gang , Ding Yong , Yang Xuejun , Chen Feng . Recent Advance of Palladium-Catalyzed Cross-Coupling Reactions of Organoalanes with Electrophiles Reagents[J]. Chinese Journal of Organic Chemistry, 2018 , 38(4) : 802 -811 . DOI: 10.6023/cjoc201709041
[1] (a) Tucker. C. E.; Vries, de J. G. Top. Catal. 2002, 19, 111.
(b) Nicolaou, K. C.; Bulger, P. G.; Sarlah, D. Angew. Chem., Int. Ed. 2005, 44, 4442.
(c) Johnson, J. B.; Rovis, T. Angew. Chem., Int. Ed. 2008, 47, 840.
(d) Torborg, C.; Beller, M. Adv. Synth. Catal. 2009, 351, 3027.
(e) Wu, X.-F.; Anbarasan, P.; Neumann, H.; Beller, M. Angew. Chem., Int. Ed. 2010, 49, 9047.
(f) Li, H.-B.; Johansson Seechurn, C. C. C.; Colacot, T. J. ACS Catal. 2012, 2, 1147.
(g) Sun, F.-Y.; Lv, L.-L.; Huang, M.; Zhou, Z.-H.; Fang, X.-D. Org. Lett. 2014, 16, 5024.
(h) Greco, R.; Goessler, W.; Cantillo, D.; Kappe, C. O. ACS Catal. 2015, 5, 1303.
(i) Ruiz-Castillo, P.; Buchwald, S. L. Chem. Rev. 2016, 116, 12564.
(j) Jedinak, L.; Zatopkova, R.; Zemankova, H.; Sustkova, A.; Cankar, P. J. Org. Chem. 2017, 82, 157.
(k) Liu, C.-W.; Liu, Y.-M.; Liu, R.-Z.; Lalancette, R.; Szostak, R.; Szostak, M. Org. Lett. 2017, 19, 1434.
(l) Halima, T. B.; Vandavasi, J. K.; Shkoor, M.; Newman, S. G. ACS Catal. 2017, 7, 2176.
[2] (a) Yu, D.-G.; Shi, Z.-J. Angew. Chem., Int. Ed. 2011, 50, 7079.
(b) Hirner, J. J.; Blum, S. A. Organometallics 2011, 30, 1299.
(c) Greene, M. A.; Yonova, I. M.; Williams, F. J.; Jarvo, E. R. Org. Lett. 2012, 14, 4293.
(d) Zhang, X.-Q.; Wang, Z.-X. Synlett 2013, 24, 2081.
(e) Everson, D. A.; Buonomo, J. A.; Weix, D. J. Synlett 2014, 25, 233.
(f) Li, Q.-H.; Ding, Y.; Yang, X. J. Chin. Chem. Lett. 2014, 25, 1296.
(g) Magano, J.; Monfette, S. ACS Catal. 2015, 5, 3120.
(h) Tarui, A.; Shinohara, S.; Sato, K.; Omote, M.; Ando, A. Org. Lett. 2016, 18, 1128.
(i) Li, Q.-H.; Ding, Y.; Zhang, G.; Zhang, Z.; Mo, S. Chin. J. Org. Chem. 2016, 36, 83(in Chinese). (李清寒, 丁勇, 张刚, 张震, 莫松, 有机化学, 2016, 36, 83.)
(j) Matsubara, K.; Yamamoto, H.; Miyazaki, S.; Inatomi, T.; Nonaka, K.; Koga, Y.; Yamada, Y.; Veiros, L. F.; Kirchner, K. Organometallics 2017, 36, 255.
[3] (a) Kang, S.-K.; Yamaguchi, T.; Kim, T.-H.; Ho, P.-S. J. Org. Chem. 1996, 61, 9082.
(b) Mao, Z.-F.; Wang, Z.; Xu, Z.-Q.; Huang, F.; Yu, Z.-K.; Wang, R. Org. Lett. 2012, 14, 3854.
(c) Hornillos, V.; Perez, M.; Fananas-Mastral, M.; Feringa, B. L. J. Am. Chem. Soc. 2013, 135, 2140.
(d) Santandrea, J.; Bedard, A. C.; Collins, S. K. Org. Lett. 2014, 16, 3892.
(e) Anima Bose, A.; Mal, P. J. Org. Chem. 2015, 80, 11219.
(f) Ding, S.-Y.; Xu, L.; Li, P.-F. ACS Catal. 2016, 6, 1329.
(g) Singh, S. K.; Chandna, N.; Jain, N. Org. Lett. 2017, 19, 1322.
(h) Sahoo, H.; Mukherjee, S.; Grandhi, G. S.; Selvakumar, J.; Baidya, M. J. Org. Chem. 2017, 82, 2764.
(i) Li, Q.-H.; Ding, Y.; Zhang, G.; Zhang, Z.; Mo, S. Curr. Org. Synth. 2017, 14, 462.
[4] (a) Baba, S.; Negishi, E.-I. J. Am. Chem. Soc. 1976, 98, 6729.
(b) Negishi, E-i.; Baba, S. J. Chem. Soc., Chem Commun. 1976, 596.
[5] (a) Negishi, E-i.; Zeng, X.; Tan, Z.; Qian, M.; Hu, Q.; Huang, Z. In Metal-catalyzed Cross-Coupling Reactions, Eds.:de Meijere, A.; Diederich, F., Vol. 2, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim, 2004.
(b) Haas, D.; Hammann, J. M.; Greiner, R.; Knochel, P. ACS Catal. 2016, 6, 1540.
[6] (a) Andrus, M. B.; Meredith, E. L.; Hicken, E. J.; Simmons, B. L.; Glancey, R. R.; Ma, W. J. Org. Chem. 2003, 68, 8162.
(b) Liu, B.; Zhou, W.-S. Org. Lett. 2004, 6, 71.
(c) Elkhayat, Z.; Safir, I.; Dakir, M.; Arseniyadis, S. Tetrahedron Asymmetry 2007, 18, 1589.
(d) Chanu, A.; Safir, I.; Basak, R.; Chiaroni, A.; Arseniyadis, S. Org. Lett. 2007, 9, 1351.
[7] Woodward, S.; Dagorne, S. Topics in Organometallic Chemistry, Vol. 41, Springer, Berlin, 2013, pp. 1~322. For the synthesis of organoaluminum compounds, see pp. 173~186, in the chapter on Preparation of organoalanes for organic synthesis, by Knochel, P.; Blimke, T.; Groll, K.; Chen, Y.-H.; For cross-coupling reactions of organoaluminum compounds, see pp. 267~276, in chapter on Organoaluminum couplings to carbonyls, imines, and halides, by Kolb, A.; Zezschwitz, P.
[8] Hallwachs, W.; Schafarik, A. Justus Liebigs Ann. Chem. 1859, 109, 206.
[9] Maruoka, K.; Yamamoto, H. Tetrahedron 1988, 44, 5001.
[10] Wang, C.; Xi, Z. Chem. Soc. Rev. 2007, 36, 1395.
[11] Uhl, W. Coord. Chem. Rev. 2008, 252, 1540.
[12] (a) Mo, S.; Shao, X.-B.; Zhang, G.; Li, Q.-H. RSC Adv. 2017, 7, 27248.
(b) Zhang, Z.; Shao, X.-B.; Zhang, G.; Li, Q.-H.; Li, X.-Y. Synthesis 2017, 49, 3643.
(c) Zhang, Z.; Mo, S.; Zhang, G.; Shao, X.-B.; Li, Q.-H.; Zhong, Y. Synlett 2017, 28, 611.
[13] Blîmke, T.; Chen, Y. H.; Peng, Z.; Knochel, P. Nat. Chem. 2010, 2, 313.
[14] (a) Ohta, A.; Inoue, A.; Watanabe, T. Heterocycles 1984, 22, 2317.
(b) Ohta, A.; Inoue, A.; Ohtsuka, K.; Watanabe, T. Heterocycles 1985, 23, 133.
[15] Hirota, K.; Isobe, Y.; Maki, Y. J. Chem. Soc., Perkin Trans. 1 1989, 2513.
[16] Crisp, G. T.; Papadopoulos, S. Aust. J. Chem. 1989, 42, 279.
[17] Hirota, K.; Kitade, Y.; Kanbe, Y.; Maki, Y. J. Org. Chem. 1992, 57, 5268.
[18] Mangalagiu, I.; Benneche, T.; Undheim, K. Tetrahedron Lett. 1996, 37, 1309.
[19] Blum, J.; Gelman, D.; Baidossi, W.; Shakh, E.; Rosenfeld, A.; Aizenshtat, Z.; Wassermann, B. C.; Frick, M.; Heymer, B.; Schutte, S.; Wernik, S.; Herbert, S. H. J. Org. Chem. 1997, 62, 8681.
[20] Biswas, K.; Chapron, A.; Cooper, T.; Fraser, P. K, Novak, A.; Prieto, O.; Woodward, S. Pure Appl. Chem. 2006, 78, 511.
[21] Vinogradov, A.; Woodward, S. Org. Synth. 2010, 87, 104.
[22] Cooper, T.; Novak, A.; Humphreys, L. D.; Walker, M. D.; Woodward, S. Adv. Synth. Catal. 2006, 348, 686.
[23] Conte, V.; Fiorani, G.; Floris, B.; Galloni, P.; Woodward, S. Appl. Catal. A:Gen. 2010, 381, 161.
[24] Baba, S.; Negishi, E.-I. J. Am. Chem. Soc. 1976, 98, 6729.
[25] Negishi, E-i.; Baba, S. J. Chem. Soc., Chem. Commun. 1976, 596.
[26] Negishi, E.-I.; Okukado, N.; King, A. O.; Van Horn, D. E.; Spiegel, B. I. J. Am. Chem. Soc. 1978, 100, 2254.
[27] Zeng, F.; Negishi, E.-I. Org. Lett. 2001, 3, 719.
[28] Qian, M.; Huang, Z.; Negishi, E.-I. Org. Lett. 2004, 6, 1531.
[29] Chen, Q.-Y.; He, Y.-B. Chin. J. Chem. 1990, 8, 451.
[30] Zweifel, G.; Miller, R. L. Organic Reactions, Vol. 32, John Wileys, New York, 1984, p. 375.
[31] Samaritani, S.; Signore, G.; Malanga, C.; Menicagli, R. Tetrahedron 2005, 61, 14475.
[32] Andrews, P.; Latham, C. M.; Magre, M.; Willcox, D.; Woodward, S. Chem. Commun. 2013, 49, 1488.
[33] Fang, H.; Yang, Z.-Y.; Zhang, L.-J.; Wang, W.; Li, Y.-M.; Xu, X.-L.; Zhou, S.-L. Org. Lett. 2016, 18, 6022.
[34] Feuvrie, C.; Blanchet, J.; Bonin, M.; Micouin, L. Org. Lett. 2004, 6, 2333.
[35] Wang, B.; Bonin, M.; Micouin, L. Org. Lett. 2004, 6, 3481.
[36] Ku, S.-L.; Hui, X.-P.; Chen, C.-A.; Kuo, Y.-Y.; Gau, H.-M. Chem Commun. 2007, 3847.
[37] Shu, W.-T.; Zhou, S.-L.; Gau, H.-M. Synthesis 2009, 4075.
[38] Gao, H. J.; Knochel, P. Synlett 2009, 1321.
[39] Blümke, T.; Chen, Y.-H. Peng, Z.; Knochel, P. Nat. Chem. 2010, 2, 313.
[40] Groll, K.; Blümke, T. D.; Unsinn, A.; Haas, D.; Knochel, P. Angew. Chem., Int. Ed. 2012, 51, 11157.
[41] Chen, X.; Zhou, L.-M.; Li, Y.-M.; Xie, T.; Zhou, S.-L. J. Org. Chem. 2014, 79, 230.
[42] (a) Necas, D.; Kotora, M.; Clsarova, I. Eur. J. Org. Chem. 2004, 6, 1280.
(b) Necas, D.; Drabina, P.; Sedlak, M.; Kotora, M. Tetrahedron Lett. 2007, 48, 4539.
(c) Kawamura, S.; Ishizuka, K.; Takaya, H.; Nakamura, M. Chem. Commun. 2010, 46, 6054.
(d) Kawamura, S.; Kawabata, T.; Ishizuka, K.; Nakamura, M. Chem. Commun. 2012, 48, 9376.
[43] (a) Wunderlich, S. H.; Knochel, P. Angew. Chem., Int. Ed. 2009, 48, 1501.
(b) Blümke, T. D.; Groll, K.; Karaghiosoff, K.; Knochel, P. Org Lett. 2011, 13, 6440.
(c) Zhou, S.-L.; Yang, Z.-Y.; Chen, X.; Li, Y.-M.; Zhang, L.-J.; Fang, H.; Wang, W.; Zhu, X.-C.; Wang, S. W. J. Org. Chem. 2015, 80, 6323.
(d) Shrestha, B.; Thapa, S.; Gurung, S. K.; Pike, R. A. S.; Giri, R. J. Org. Chem. 2016, 81, 787.
[44] (a) Biradar, D. B.; Gau, H.-M. Chem. Commun. 2011, 47, 10467.
(b) Biradar, D. B.; Gau, H.-M. Org. Biomol. Chem. 2012, 10, 4243.
(c) He, F.; Wang, Z.-X. Tetrahedron 2017, 73, 4450.
(d) Mo, S.; Shao, X.-B.; Zhang, G.; Li, Q.-H. RSC Adv. 2017, 7, 27243.
/
| 〈 |
|
〉 |