

Chinese Journal of Organic Chemistry >
Synthesis and Immobilization of Pyridinium N-Chloramine Precursors on PU Film for Antibacterial Application
Received date: 2017-08-27
Revised date: 2017-11-28
Online published: 2017-12-08
Supported by
Project supported by the Fundamental Research Funds for the Central Universities (Nos. DUT14RC(3)081, DUT17LK17) and the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry.
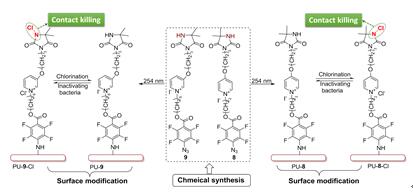
In this work, two novel pyridinium N-chloramine precursors were designed and synthesized, both of which contain perfluorophenyl azide (PFPA) unit as the photo-coupling handle. The synthetic precursors were photo immobilized on commercial PU films upon UV (254 nm) light irradiation. After exposure to diluted household bleach, the resulting PU films were rendered biocidal. Antibacterial tests showed that satisfactory was achieved for both surface modified films and that the film grafted with shorter alkyl linker-contained precursor demonstrated even higher biocidal efficacy. Non-leaching antibacterial PU materials were herein developed based on PFPA-coupling strategy, providing a simple and universal method to confer N-chloramines on inert polymer surface for antibacterial application.
Li Lingdong , Chi Xiaofang , Yan Jiawei , Zhao Zihan . Synthesis and Immobilization of Pyridinium N-Chloramine Precursors on PU Film for Antibacterial Application[J]. Chinese Journal of Organic Chemistry, 2018 , 38(4) : 955 -962 . DOI: 10.6023/cjoc201708056
[1] Kang, Z.; Jiao, Y.; Zhang, B.; Liang, J. J. Shanghai Normal Univ. (Nat. Sci. Ed.) 2012, 41, 540(in Chinese). (亢真真, 焦玉超, 张冰, 梁杰, 上海师范大学学报(自然科学版), 2012, 41, 540.)
[2] Hui, F.; Debiemme-Chouvy, C. Biomacromolecules 2013, 14, 585.
[3] Dong, A.; Wang, Y.-J.; Gao, Y.; Gao, T.; Gao G. Chem. Rev. 2017, 117, 4806.
[4] Gottardi, W.; Debabov, D.; Nagl, M. Antimicrob. Agents Chemother. 2013, 57, 1107.
[5] Jie, Z.; Yan, X.; Zhao, L.; Worley, S. D.; Liang. J. RSC Adv. 2014, 4, 6048.
[6] Li, L.; Pu, T.; Zhanel, G.; Zhao, N.; Ens, W.; Liu, S. Adv. Healthcare Mater. 2012, 1, 609.
[7] Ning, C.; Li, L.; Logsetty, S.; Ghanbar, S.; Guo, M.; Ensf, W.; Liu, S. RSC Adv. 2015, 5, 93877.
[8] Li, L.; Zhao, Y.; Zhou, H.; Ning, A.; Zhang, F.; Zhao, Z. Tetrahedron Lett. 2017, 58, 321.
[9] Li, L.; Zhou, H.; Gai, F.; Chi, X.; Zhang, F.; ZhaoZ. RSC Adv. 2017, 7, 13244.
[10] Liang, J.; Chen, Y.; Ren, X. Ind. Eng. Chem. Res. 2007, 46, 6425.
[11] Wu, L.; Liu A.; Li, Z. Fibers Polym. 2015, 16, 550.
[12] Sun, Y. Y.; Sun, G. J. Appl. Polym. Sci. 2001, 81, 1517.
[13] Liu, L.-H.; Yan, M. Acc. Chem. Res. 2010, 43, 1434.
[14] Li, L.; Li, J.; Kulkarni, A.; Liu, S. J. Mater. Chem., 2013, 1, 571.
[15] Williamson, M. R.; Black, R.; Kielty, C. Biomaterials 2006, 27, 3608.
[16] Li, L.; Chi, X.; Gai, F.; Zhou, H.; Zhang, F.; Zhao, Z. J. Appl. Polym. Sci. 2017, 134, 45323.
[17] Dastgir, S.; Coleman, K. S.; Green, M. L. H. Dalton Trans. 2011, 40, 661.
[18] Keana, J. F. W.; Cai, S. X. J. Org. Chem. 1990, 55, 3640.
[19] Xiong, J.; Xia, L.; Shentu, B.; Weng, Z. J. Appl. Polym. Sci. 2014, 131, 39812.
[20] Renaudie, L.; Narvor, C. L.; Lepleux, E.; Roger, P. Biomacromolecules 2007, 8, 679.
[21] Li, L.; Zhao, N.; Liu, S. Polymer 2012, 53, 67.
/
| 〈 |
|
〉 |