

Chinese Journal of Organic Chemistry >
Trityl Ion-Mediated Oxidative C—H Alkynylation of 1, 2-Dihydroquinolines
Received date: 2018-03-01
Revised date: 2018-03-16
Online published: 2018-04-13
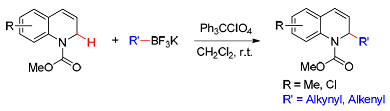
An efficient synthesis of α-substituted 1, 2-dihydroquinoline compounds through the oxidative C—H functionalization of N-acyl-dihydroquinoline with diverse organoboron reagents mediated by triphenylcarbium perchlorate (Ph3CClO4) is reported. The reaction exhibits good functional group tolerance, allowing for C—H alkynylation and alkenylation proceeding smoothly in good yields.
Liu Ziqiang , Zhao Ran , He Ni , Li Wei . Trityl Ion-Mediated Oxidative C—H Alkynylation of 1, 2-Dihydroquinolines[J]. Chinese Journal of Organic Chemistry, 2018 , 38(5) : 1261 -1266 . DOI: 10.6023/cjoc201803001
[1] (a) Michael, J. P. Nat. Prod. Rep. 2007, 24, 223.
(b) Michael, J. P. Nat. Prod. Rep. 2008, 25, 166.
(c) Welsch, M. E.; Snyder, S. A.; Stockwell, B. R. Curr. Opin. Chem. Biol. 2010, 14, 347.
[2] (a) Kouznetsov, V.; Palma, A.; Ewert, C.; Varlamov, A. J. Heterocycl. Chem. 1998, 35, 761.
(b) Sridharan, V.; Suryavanshi, P. A.; Menéndez, J. C. Chem. Rev. 2011, 111, 7157.
(c) Katritzky, A. R.; Rachwal, S.; Rachwal, B. Tetrahedron 1996, 52, 15031.
[3] (a) Takamura, M.; Funabashi, K.; Kanai, M.; M. Shibasaki, J. Am. Chem. Soc. 2001, 123, 6801.
(b) Yamaoka, Y.; Miyabe, H.; Takemoto, Y. J. Am. Chem. Soc. 2007, 129, 6686.
[4] (a) Graham, T. J. A.; Shields, J. D.; Doyle, A. G. Chem. Sci. 2011, 2, 980.
(b) Kodama, T.; Moquist, P. N.; Schaus, S. E. Org. Lett. 2011, 13, 6316.
(c) Sun, S.; Mao, Y.; Lou, H.; Liu, L. Chem. Commun. 2015, 51, 10691.
(d) Volla, C. M. R.; Fava, E.; Atodiresei, L.; Rueping, M. Chem. Commun. 2015, 51, 15788.
(e) Berti, F.; Malossi, F.; Marchettib, F.; Pineschi, M. Chem. Commun. 2015, 51, 13694.
[5] (a) Trost, B. M. Acc. Chem. Res. 2002, 35, 695.
(b) Wender, P. A.; Verma, V. A.; Paxton, T. J.; Pillow, T. H. Acc. Chem. Res. 2008, 41, 40.
[6] (a) Murahashi, S.; Zhang, D. Chem. Soc. Rev. 2008, 37, 1490.
(b) Liu, C.; Zhang, H.; Shi, W.; Lei, A. Chem. Rev. 2011, 111, 1780.
(c) Li, C. J. Acc. Chem. Res. 2009, 42, 335.
(d) Sun, C. L.; Li, B. J.; Shi, Z. J. Chem. Rev. 2011, 111, 1293.
(e) Zhang, C.; Tang, C.; Jiao, N. Chem. Soc. Rev. 2012, 41, 3464.
(f) Cho, S. H.; Kim, J. Y.; Kwak, J.; Chang, S. Chem. Soc. Rev. 2011, 40, 5068.
(g) Liu, Z.; Chen, L.; Li, J.; Liu, K.; Zhao, J.; Xu, M.; Feng, L.; Wan, R.; Li, W.; Liu, L. Org. Biomol. Chem, 2017, 15, 7600.
(h) Yeung, C. S.; Dong, V. M. Chem. Rev. 2011, 111, 1215.
(i) Liu, X.; Sun, S.; Meng, Z.; Lou, H.; Liu, L. Org. Lett. 2015, 17, 2396.
(j) Xie, Z.; Zan, X.; Sun, S.; Pan, X.; Liu, L. Org. Lett. 2016, 18, 3944.
(k)Wang, G., Mao, Y., Liu, L. Org. Lett. 2016, 18, 6476.
(l) Zhang, Q.; Lv, J.; Luo, S. Acta Chim. Sinica 2016, 74, 61(in Chinese). (张启超, 吕健, 罗三中, 化学学报, 2016, 74, 61.)
(m) Qin, Y.; Zhu, L.; Luo, S. Chem. Rev. 2017, 117, 9433.
[7] (a) Murahashi, S.-I.; Komiya, N.; Terai, H.; Nakae, T. J. Am. Chem. Soc. 2003, 125, 15312.
(b) Murahashi, S.-I.; Nakae, T.; Terai, H.; Komiya, N. J. Am. Chem. Soc. 2008, 130, 11005.
(c) Boess, E.; Sureshkumar, D.; Sud, A.; Wirtz, C.; Farès, C.; Klussmann, M. J. Am. Chem. Soc. 2011, 133, 8106.
(d) Boess, E.; Schmitz, C.; Klussmann, M. J. Am. Chem. Soc. 2012, 134, 5317.
(e) Li, Z.; Li, C. J. J. Am. Chem. Soc. 2004, 126, 11810.
(f) Li, Z.; Yu, R.; Li, H. Angew. Chem., Int. Ed. 2008, 47, 7497.
(g) Yang, F.; Li, J.; Xie, J.; Huang, Z. Z. Org. Lett. 2010, 12, 5214.
(h) Muramatsu, W.; Nakano, K.; Li, C. J. Org. Lett. 2013, 15, 3650.
(i) Li, Z.; Li, C.-J. Org. Lett. 2004, 6, 4997.
(j) Zhang, G.; Zhang, Y.; Wang, R. Angew. Chem., Int. Ed. 2011, 50, 10429.
[8] (a) Guo, C.; Song, J.; Luo, S.-W.; Gong, L.-Z. Angew. Chem., Int. Ed. 2010, 49, 5558.
(b) Li, Z.; MacLeod, P. D.; Li, C.-J. Tetrahedron:Asymmetry 2006, 17, 590.
(c) Yang, Q.; Zhang, L.; Ye, C.; Luo, S.; Wu, L.-Z. Tung, C.-H. Angew. Chem., Int. Ed. 2017, 56, 3694.
(d) Neel, A. J.; Hehn, J. P.; Tripet, P. F.; Toste, F. D. J. Am. Chem. Soc. 2013, 135, 14044.
(e) Zhang, G.; Ma, Y.; Wang, S.; Zhang, Y.; Wang, R. J. Am. Chem. Soc. 2012, 134, 12334.
(f) Zhang, G.; Ma, Y.; Wang, S.; Kong, W.; Wang, R. Chem. Sci. 2013, 4, 2645.
(g) Bergonzini, G.; Schindler, C. S.; Wallentin, C.-J.; Jacobsen, E. N.; Stephenson, C. R. J. Chem. Sci. 2014, 5, 112.
[9] (a) Pintér, Á.; Sud, A.; Sureshkumar, D.; Klussmann, M. Angew. Chem., Int. Ed. 2010, 49, 5004.
(b) Liu, X.; Meng, Z.; Li, C.; Lou, H.; Liu, L. Angew. Chem., Int. Ed. 2015, 54, 6012.
(c) Richter, H.; Fröhlich, R.; Daniliuc, C.-G.; García Mancheño, O. Angew. Chem., Int. Ed. 2012, 51, 8656.
(d) Richter, H.; García Mancheño, O. Eur. J. Org. Chem. 2010, 4460.
(e) Ghobrial, M.; Harhammer, K.; Mihovilovic, M. D.; Schnürch, M. Chem. Commun. 2010, 46, 8836.
(f) Ghobrial, M.; Schnürch, M.; Mihovilovic, M. D. J. Org. Chem. 2011, 76, 8781.
(g) Liu, X.; Sun, B.; Xie, Z.; Qin, X.; Liu, L.; Lou, H. J. Org. Chem. 2013, 78, 3104.
(h) Sun, S.; Li, C.; Floreancig, P. E.; Lou, H.; Liu, L. Org. Lett. 2015, 17, 1684.
(i) Long, H.; Wang, G.; Lu, R.; Xu, M.; Zhang, K.; Qi, S.; He, Y.; Bu, Y.; Liu, L. Org. Lett. 2017, 19, 2146.
[10] (a) Xie, Z.; Liu, L.; Chen, W.; Zheng, H.; Xu, Q.; Yuan, H.; Lou, H. Angew. Chem., Int. Ed. 2014, 53, 3904.
(b) Chen, W.; Xie, Z.; Zheng, H.; Lou, H.; Liu, L. Org. Lett. 2014, 16, 5988.
(c) Wan, M.; Meng, Z.; Lou, H.; Liu, L. Angew. Chem., Int. Ed. 2014, 53, 13845.
[11] (a) Kim, H.; MacMillan, D. W. C. J. Am. Chem. Soc. 2008, 130, 398.
(b) Vo, C.-V. T.; Mitchell, T. A.; Bode, J. W. J. Am. Chem. Soc. 2011, 133, 14082.
(c) Fujiwara, Y.; Domingo, V.; Seiple, I. B.; Gianatassio, R.; Del Bel, M.; Baran, P. S. J. Am. Chem. Soc. 2011, 133, 3292.
(d) Nielsen, D. K.; Doyle, A. G. Angew. Chem., Int. Ed. 2011, 50, 6056.
/
| 〈 |
|
〉 |