

Chinese Journal of Organic Chemistry >
Supramolecular Assembly Based on the Novel Sail-Boat-Shaped Self-Complexes
Received date: 2018-10-01
Revised date: 2018-10-29
Online published: 2018-11-26
Supported by
Project supported by the National Natural Science Foundation of China (No. 21072066), the Key Project on the Integration of Industry, Education and Research of Guangdong Province (No. 2012B090700003), and the Foundation of Young Teachers of South China Normal University (No.14KJ02).
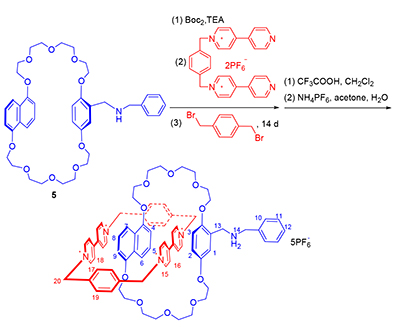
Owing to the unique macrocyclic-cavity structure of crown ether and the capability of complexing with guest molecules, our group successfully designed and synthesized a novel Sailboat-Shaped self-complex by using intramolecular hydrogen bonds, which are able to switch its configuration during the different pH value. Furthermore, a novel catenane which based on the sailboat-shaped self-complex was obtained by using intermolecular charge transfer interaction and through the template-directed method, and its structure was identified by 1H NMR, 13C NMR, HRMS and 1H-1H NOESY. It is hoped that it can be successfully controlled by the pH value conversion.
Key words: self-complexes; charge transfer interaction; catenane
Zhang Shilong , Jiang Lasheng . Supramolecular Assembly Based on the Novel Sail-Boat-Shaped Self-Complexes[J]. Chinese Journal of Organic Chemistry, 2019 , 39(3) : 720 -726 . DOI: 10.6023/cjoc201810001
[1] Desiraju, G. R. Acc. Chem. Res. 2002, 35, 565.
[2] Xiao, T. S.; Fan, J. S.; Zhou, H.; Lin, Q. S.; Yang, D. W. Angew. Chem., Int. Ed. 2016, 55, 6869.
[3] He, H. H.; Ye, Z. W.; Zheng, Y.; Xu, X.; Guo, C. L.; Xiao, Y.; Yang, W.; Qian, X. H.; Yang, Y. J. Chem. Commun. 2018, 54, 2842.
[4] Stoddart, J. F. Angew. Chem., Int. Ed., 2017, 56, 11094.
[5] Pressman, B. C.; Harris, H. J.; Jagger, W. S.; Jonson, J. H. Proc. Natl. Acad. Sci. U. S. A. 1967, 58, 32
[6] Yan, Z. Q.; Huang, Q. F.; Liang, W. T.; Yu, X. K.; Zhou, D. Y.; Wu, W. H.; Chruma, J. J.; Yang, C. Org. Lett. 2017, 19, 898.
[7] Chen, Y.; Huang, F. H.; Li, Z. T.; Liu, Y. Sci. China Chem. 2018, 61, 979.
[8] Jiao, Y.; Zhang, X. Acta Chim. Sinica 2018, 76, 659(in Chinese). (焦阳, 张希, 化学学报, 2018, 76, 659.)
[9] Guo, Q. H.; Zhao, L.; Wang, M. X. Angew. Chem., Int. Ed. 2015, 54, 8386.
[10] Chen, H. Q.; Fan, J. Z.; Hu, X. S.; Ma, J. W.; Wang, S. L.; Li, J.; Yu, Y. H.; Jia, X. S.; Li, C. J. Chem. Sci. 2015, 6, 197.
[11] Rudkevich, D. M.; Mercer-Chalmers, J. D.; Verboom, W.; Ungaro, R.; de Jong, F.; Reinhoudt, D. N. J. Am. Chem. Soc. 1995, 117, 6124.
[12] Rose, A. S.; Dennis, M. D.; George, W. G. J. Am. Chem. Soc. 1982, 104, 625.
[13] Zhu, S. S.; Carroll, P. J.; Swager, T. M. J. Am. Chem. Soc. 1996, 118, 8713.
[14] Jiang, F.; Chen, M.; Liang, J.; Gao, Z.; Tang, M.; Xu, Z.; Peng, B.; Zhu, S.; Jiang, L. Eur. J. Org. Chem. 2016, 3310.
[15] Fan, J. Q.; Jiang, L. S.; Zhang, M.; Wei, N.; Feng, Y. F.; Wang, H. Chin. Chem. Lett. 2008. 19, 513.
[16] Steed, J. W.; Atwood, J. L. Supramolecular Chemistry, Trans. by Zhao, Y.-P.; Sun, Z., Chemical Industry Press, Beijing, 2006(in Chinese). (Steed, J. W.; Atwood, J. L. 超分子化学, 赵耀鹏, 孙震译, 化学工业出版社, 北京, 2006.)
[17] Gururaja, T. L.; Ramasubbu, N.; Levine, M. J. Lett. Pept. Sci. 1996, 3, 79.
[18] Guo, Q. H.; Zhao, L.; Wang, M. X. Angew. Chem., Int. Ed. 2015, 54, 8386.
[19] Basu, S.; Coskun, A.; Friedman, D. C.; Olson, M. A.; Bentez, D.; Tkatchouk, E.; Barin, G.; Yang, J.; Fahrenbach, A. C.; Goddard Ⅲ, W. A.; Stoddart, J. F. Chem.-Eur. J. 2011, 17, 2107.
/
| 〈 |
|
〉 |