

Chinese Journal of Organic Chemistry >
Two New Isocoumarins Isolated from a Mangrove-Derived Fungus Penicillium citrinum HL-5126
Received date: 2018-12-05
Revised date: 2018-12-29
Online published: 2019-01-18
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21462015, 31760093), the National Natural Science Foundation of Hainan Province (No. 218MS045), the Program for Innovative Research Team in University (No. IRT-16R19).
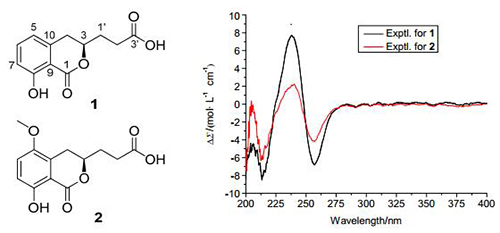
Two new isocoumarins derivatives, penicimarins J (1) and K (2), were isolated from the mangrove-derived fungus Penicillium citrinum HL-5126. Their structures were elucidated through extensive 1D NMR, 2D NMR and HR-ESI-MS spectroscopic analyses. The absolute configurations of 1 and 2 were determined by comparison of their circular dichroism (CD) spectra with the literature. Compounds 1 and 2 showed inhibitory activities against α-glucosidase with the IC50 values of 18.37 and 25.86 μg/mL, respectively.
Mei Rongqing , Huang Guolei , Wang Bin , Bai Meng , Luo Youping , Chen Guangying , Zheng Caijuan . Two New Isocoumarins Isolated from a Mangrove-Derived Fungus Penicillium citrinum HL-5126[J]. Chinese Journal of Organic Chemistry, 2019 , 39(5) : 1479 -1482 . DOI: 10.6023/cjoc201812008
[1] Blunt, J. W.; Carroll, A. R.; Copp, B. R.; Davis, R. A.; Keyzer, R. A.; Prinsep, M. R. Nat. Prod. Rep. 2018, 35(1), 8.
[2] Sun, Y. L.; Bao, J.; Liu, K. S.; Zhang, X. Y.; He, F.; Nong, X. H.; Qi, S. H. Planta Med. 2013, 79(15), 1474.
[3] Qi, J.; Shao, C. L.; Liu, M.; Qi, X.; Wang, C. Y. Chem. Nat. Compd. 2014, 50(3), 568.
[4] Asiri, I. A. M.; Badr, J. M.; Youssef, D. T. A. Phytochem. Lett. 2015, 13, 53.
[5] Zheng, C. J.; Huang, G. L.; Xu, Y. Song, X. M.; Yao, J. Liu, H. Wang, R. P.; Song X. P. Nat. Prod. Res. 2016, 30, 821.
[6] Ha, T. M.; Ko, W.; Lee, S. J.; Kim, Y. C.; Son, J. Y.; Sohn, J. H.; Yim, J. H.; Oh, H. Mar. Drugs 2017, 15(9), 282.
[7] Zhou, X. M.; Zheng, C. J.; Chen, G. Y.; Song, X. P.; Han, C. R.; Li, G. N.; Fu, Y. H.; Chen, W. H.; Niu, Z. G. J. Nat. Prod. 2014, 77, 2021.
[8] Liao, H. X.; Sun, D. W.; Zheng, C. J.; Wang, C. Y. Nat. Prod. Res. 2017, 31(14), 1640.
[9] He, K. Y.; Zhang, C.; Duan, Y. R.; Huang, G. L.; Yang, C. Y.; Lu, X. R.; Zheng, C. J; Chen, G. Y. J. Antibiot. 2017, 70, 823.
[10] Zheng, C. J; Wu, L. Y.; Li, X. B.; Song, X. M. Niu, Z. G.; Song, X. P.; Chen, G. Y.; Wang, C. Y. Helv. Chim. Acta 2015, 98, 368.
[11] Zheng, C. J.; Huang, G. L.; Xu, Y.; Song, X. M.; Yao, J. Liu, H.; Wang, R. P.; Song, X. P. Nat. Prod. Res. 2016, 30, 821.
[12] Zheng, C. J.; Bai, M. Zhou, X. M.; Huang, G. L.; Shao, T. M.; Luo, Y. P.; Niu, Z. G.; Niu, Y. Y.; Chen, G. Y.; Han, C. R. J. Nat. Prod. 2018, 81(4), 1045.
[13] Zheng, C. J.; Liao, H. X.; Mei, R. Q.; Huang, G. L.; Yang, L. J.; Zhou, X. M.; Shao, T. M.; Chen, G. Y.; Wang, C. Y. Nat. Prod. Res. 2019, 33, 1127.
[14] Huang, G. L.; Zhou, X. M.; Bai, M.; Liu, Y. X.; Zhao, Y. L.; Luo, Y. P.; Niu, Y. Y.; Zheng, C. J.; Chen, G. Y. Mar. Drugs 2016, 14(10), 177.
[15] Choukchou-Braham, N.; Asakawa, Y.; Lepoittevin, J. P. Tetrahedron Lett. 1994, 35, 3949.
[16] Rivera-Chávez, J.; Figueroa, M.; González, M. D. C.; Glenn, A. E.; Mata, R. J. Nat. Prod. 2015, 78, 730.
[17] Pierce, C. G.; Uppuluri, P.; Teistan, A. R.; Wormley, J. F. L.; Mowat, E.; Ramage, G.; Lopez-ribot, J. L. Nat. Protoc. 2008, 3, 1494.
/
| 〈 |
|
〉 |