

Chinese Journal of Organic Chemistry >
Copper-Catalyzed Aerobic Oxidation Strategy: A Concise Route to Isatin
Received date: 2019-03-14
Online published: 2019-06-12
Supported by
the National Natural Sciences Foundation of China(21672144)
A copper-catalyzed decarbonylation cyclization to form isatins using oxygen as terminal oxidant is developed. This complementary way offers a new protocol for the synthesis of isatins through C(sp3)—H bond functionalization in Cu/O2/Co system. This system shows good reactivity and compatibility. Both electron-rich and electron-deficient functional groups can be tolerated. A postulated mechanism is proposed based on mechanistic studies and previous reports.
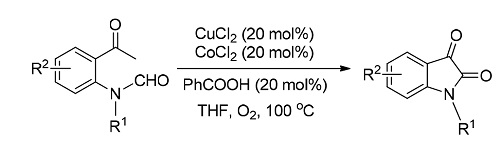
Key words: Cu catalyst; C—H bond activation; isatin; decarbonylation; oxygen
Muhammad Siddique Ahmad , Yamin Zhu , Yunlong Guo , Saisai Zhang , Zengming Shen . Copper-Catalyzed Aerobic Oxidation Strategy: A Concise Route to Isatin[J]. Chinese Journal of Organic Chemistry, 2019 , 39(11) : 3244 -3249 . DOI: 10.6023/cjoc201903024
| [1] | (a) Sumpter, W. C. Chem. Rev. 1944, 34, 393. |
| [1] | (b) da Silva, J. F. M.; Garden, S. J.; Pinto, A. C. J. Braz. Chem. Soc. 2001, 12, 273. |
| [2] | (a) Ratan, B. T.; Anand, B.; Yogeeswari, P.; Sriram, D. Bioorg. Med. Chem. Lett. 2005, 15, 4451. |
| [2] | (b) Raj, A.; Raghunathan, R.; Sridevikumaria, M. R.; Raman, N. Bioorg. Med. Chem. 2003, 11, 407. |
| [2] | (c) Verma, M.; Pandeya, S. N.; Singh, K. N.; Stables, J. P. Acta Pharm. 2004, 54, 49. |
| [2] | (d) Jiang, T.; Kuhen, K. L.; Wolff, K.; Yin, H.; Bieza, K.; Caldwell, J.; Bursulaya, B.; Tuntlad, T.; Zhang, K.; Karanewsky, D.; He, Y. Bioorg. Med. Chem. Lett. 2006, 16, 2109. |
| [2] | (e) Aboul-Fadl, T.; Bin-Jubair, F. A. S. Int. J. Res. Pharm. Sci. 2010, 1, 113. |
| [2] | (f) Sharma, S.; Gupta, M. K.; Saxena, A. K.; Bedi, P. M. S. Bioorg. Med. Chem. 2015, 23, 7165. |
| [2] | (g) Harbinder, S.; Jatinder, V. S.; Gupta, M. K.; Sharma, S.; Nepali, K.; Bedi, P. M. S. Bioorg. Med. Chem. Lett. 2017, 27, 3974. |
| [3] | Sandmeyer T. Helv. Chim. Acta 1919, 2 234. |
| [4] | (a) Stollé, R. Ber. Dtsch. Chem. Ges. 1913, 46, 3915. |
| [4] | (b) Stollé, R. J. Prakt. Chem. 1922, 106, 137. |
| [5] | (a) Martinet, J. Compt. Rend. 1918, 166, 85. |
| [5] | (b) Bonnefoy, J.; Martinet, J. Compt. Rend. 1921, 172, 220. |
| [6] | Xie Y. Chem. Commun. 2016, 52 12372. |
| [7] | (a) Sun, J.; Liu, B.; Xu, B. RSC Adv. 2013, 3, 5824. |
| [7] | (b) Liu, T.; Yang, H.; Jiang, Y.; Fu, H. Adv. Synth. Catal. 2013, 355, 1169. |
| [7] | (c) Tang, B.-X.; Song, R.-J.; Wu, C.-Y.; Liu, Y.; Zhou, M.-B.; Wei, W.-T.; Deng, G.-B.; Yin, D.-L.; Li, J.-H. J. Am. Chem. Soc. 2010, 132, 8900. |
| [7] | (d) Liu, T.; Yang, H.; Jiang, Y.; Fu, H. Adv. Synth. Catal. 2013, 355, 1169. |
| [7] | (e) Sun, J.; Liu, B.-X.; Xu, B. RSC Adv. 2013, 3, 5824. |
| [7] | (f) Ilangovan, A.; Satish, G. Org. Lett. 2013, 15, 5726. |
| [7] | (g) Huang, P. C.; Gandeepan, P.; Cheng, C. H. Chem. Commun. 2013, 49, 8540. |
| [7] | (h) Li, J.; Zheng, Y.; Yu, X. L.; Lv, S. Y.; Wang, Q. T.; Hai, L.; Wu Y. RSC Adv. 2015, 5, 103280. |
| [7] | (i) Liu, Z.; Zhang, J.; Chen, S.; Shi, E.; Xu, Y.; Wan, X. Angew. Chem., Int. Ed. 2012, 51, 3231. |
| [7] | (j) Meng, Q.; Wang, F.; Li, M. J. Mol. Model. 2013, 19, 2225. |
| [8] | Liao Y.-Y.; Gao Y.-C.; Zheng W.; Tang R.-Y. Adv. Synth. Catal. 2018, 360 3391. |
| [9] | Lollar C. T.; Krenek K. M.; Bruemmer K. J.; Lippert A. R. Org. Biomol. Chem. 2014, 12 406. |
| [10] | (a) Satish, G.; Polu, A.; Ramar, T.; Ilangovan, A. J. Org. Chem. 2015, 80, 5167. |
| [10] | (b) Reddy, M. R.; Rao, N. N.; Ramakrishna, K.; Meshram, H. M. Tetrahedron Lett. 2014, 55, 4758. |
| [10] | (c) Ilangovan, A.; Satish, G. J. Org. Chem. 2014, 79, 4984. |
| [10] | (d) Gao, F. F.; Xue, W. J.; Wang, J. G.; Wu, A. X. Tetrahedron 2014, 70, 4331. |
| [11] | (a) Huang, P. C.; Gandeepan, P.; Cheng, C. H. Chem. Commun. 2013, 49, 8540. |
| [11] | (b) Wu, H.; Zhang, Z. G.; Liu, Q. F.; Liu, T. X.; Ma, N. N.; Zhang, G. S. Org. Lett. 2018, 20, 2897. |
| [11] | (c) Salvanna, N.; Reddy, L. M.; Kumar, R. A.; Das, B. ChemistrySelect 2018, 3, 8019. |
| [12] | Nobrega J. A.; Goncalves S. M. C.; Peppe C. Synth. Commun. 2002, 32 3711. |
| [13] | Shekhar A. C.; Kumar A. R.; Sathaiah G.; Paul V. L.; Sridhar M.; Rao P. S. Tetrahedron Lett. 2009, 50 7099. |
| [14] | Kirincich S. J.; Xiang J.; Green N.; Tam S.; Yang H. Y.; Shim J.; Clark J. D.; McKew J. C. Bioorg. Med. Chem. 2009, 17 4383. |
| [15] | Luo J. F.; Gao S. S.; Ma Y. R.; Ge G. P. Synlett 2018, 29 969. |
| [16] | Ji H. H.; Zhu Y. Z.; Shao Y.; Liu J.; Yuan Y.; Jia X. D. J. Org. Chem. 2017, 82 9859. |
| [17] | Wang H. Y.; Wang K. Y.; Man Y. Q.; Gao X. N.; Yang L. M.; Ren Y. F.; Li N.; Tang B.; Zhao G. Adv. Synth. Catal. 2017, 359 3934. |
| [18] | Bredenkampa A.; Mohrb F.; Kirsch S. F. Synthesis 2015, 47 1937. |
| [19] | Zhang C.; Li S.; Filip B.; Richmond L.; Ye X.; Jiang Z. ACS Catal. 2016, 6 10 6853. |
| [20] | Salvanna N.; Ramesh P.; Kumarc K. S.; Das B. New J. Chem. 2017, 41 13754. |
/
| 〈 |
|
〉 |