Chinese Journal of Organic Chemistry ›› 2019, Vol. 39 ›› Issue (11): 3244-3249.DOI: 10.6023/cjoc201903024 Previous Articles Next Articles
AhmadMuhammad Siddique, 主亚敏, 郭云龙, 张赛赛, 沈增明*( )
)
收稿日期:2019-03-14
发布日期:2019-06-12
通讯作者:
沈增明
E-mail:shenzengming@sjtu.edu.cn
基金资助:
Ahmad Muhammad Siddique, Zhu Yamin, Guo Yunlong, Zhang Saisai, Shen Zengming*( )
)
Received:2019-03-14
Published:2019-06-12
Contact:
Shen Zengming
E-mail:shenzengming@sjtu.edu.cn
Supported by:Share
Ahmad Muhammad Siddique, Zhu Yamin, Guo Yunlong, Zhang Saisai, Shen Zengming. Copper-Catalyzed Aerobic Oxidation Strategy: A Concise Route to Isatin[J]. Chinese Journal of Organic Chemistry, 2019, 39(11): 3244-3249.
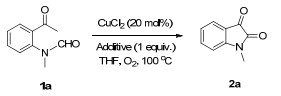 | ||||
| Entry | Cat. | Additive | Time/h | Yieldb/% |
| 1 | CuCl2 | None | 49 | 34 |
| 2 | CuCl | None | 110 | 23 |
| 3 | CuBr | None | 110 | 17 |
| 4 | CuI | None | 82 | N.R. |
| 5 | CuSCN | None | 82 | N.R. |
| 6 | CuCN | None | 82 | N.R. |
| 7 | Cu2O | None | 110 | Trace |
| 8 | Cu(OAc)2 | None | 66 | N.R. |
| 9 | CuCl2 | ZnCl2 (1 equiv.) | 49 | 40 |
| 10 | CuCl2 | AlCl3 (1 equiv.) | 49 | N.R. |
| 11 | CuCl2 | BF3·Et2O (1 equiv.) | 67 | N.R. |
| 12 | CuCl2 | FeCl3 (1 equiv.) | 67 | N.R. |
| 13 | CuCl2 | PdCl2 (1 equiv.) | 67 | N.R. |
| 14 | CuCl2 | Cu(ClO4)·6H2O (1 equiv.) | 67 | N.R. |
| 15 | CuI | CoSO4 (1 equiv.) | 59 | 45 |
| 16 | CuSCN | CoCl2 (1 equiv.) | 59 | 62 |
| 17 | CuCN | Yb(OTf)3 (1 equiv.) | 48 | N.R. |
| 18 | Cu2O | Ni(OAc)2·4H2O (1 equiv.) | 65 | 16 |
| 19 | Cu(OAc)2 | Co(NO3)2·6H2O (1 equiv.) | 65 | Trace |
| 20 | CuCl2 | Cu(OAc)2·4H2O (1 equiv.) | 65 | N.R. |
| 21 | CuCl2 | LiCl (1 equiv.) | 49 | N.R. |
| 22 | CuCl2 | CuCl2(1 equiv.) | 49 | 23 |
| 23 | CuCl2 | CuCl2(1 equiv.)+ PivOH (1 equiv.) | 58 | 47 |
| 24 | CuCl2 | CuCl2(1 equiv.)+ PhCOOH (1 equiv.) | 57 | 69 |
| 25 | CuCl2 | CuCl2(20 mol%)+PhCOOH (20 mol%) | 84 | 78 |
| 26 | None | CoCl2 (1 equiv.)+PhCOOH (20 mol%) | 77 | N.R. |
 | ||||
| Entry | Cat. | Additive | Time/h | Yieldb/% |
| 1 | CuCl2 | None | 49 | 34 |
| 2 | CuCl | None | 110 | 23 |
| 3 | CuBr | None | 110 | 17 |
| 4 | CuI | None | 82 | N.R. |
| 5 | CuSCN | None | 82 | N.R. |
| 6 | CuCN | None | 82 | N.R. |
| 7 | Cu2O | None | 110 | Trace |
| 8 | Cu(OAc)2 | None | 66 | N.R. |
| 9 | CuCl2 | ZnCl2 (1 equiv.) | 49 | 40 |
| 10 | CuCl2 | AlCl3 (1 equiv.) | 49 | N.R. |
| 11 | CuCl2 | BF3·Et2O (1 equiv.) | 67 | N.R. |
| 12 | CuCl2 | FeCl3 (1 equiv.) | 67 | N.R. |
| 13 | CuCl2 | PdCl2 (1 equiv.) | 67 | N.R. |
| 14 | CuCl2 | Cu(ClO4)·6H2O (1 equiv.) | 67 | N.R. |
| 15 | CuI | CoSO4 (1 equiv.) | 59 | 45 |
| 16 | CuSCN | CoCl2 (1 equiv.) | 59 | 62 |
| 17 | CuCN | Yb(OTf)3 (1 equiv.) | 48 | N.R. |
| 18 | Cu2O | Ni(OAc)2·4H2O (1 equiv.) | 65 | 16 |
| 19 | Cu(OAc)2 | Co(NO3)2·6H2O (1 equiv.) | 65 | Trace |
| 20 | CuCl2 | Cu(OAc)2·4H2O (1 equiv.) | 65 | N.R. |
| 21 | CuCl2 | LiCl (1 equiv.) | 49 | N.R. |
| 22 | CuCl2 | CuCl2(1 equiv.) | 49 | 23 |
| 23 | CuCl2 | CuCl2(1 equiv.)+ PivOH (1 equiv.) | 58 | 47 |
| 24 | CuCl2 | CuCl2(1 equiv.)+ PhCOOH (1 equiv.) | 57 | 69 |
| 25 | CuCl2 | CuCl2(20 mol%)+PhCOOH (20 mol%) | 84 | 78 |
| 26 | None | CoCl2 (1 equiv.)+PhCOOH (20 mol%) | 77 | N.R. |
| [1] |
(a) Sumpter, W. C. Chem. Rev. 1944, 34, 393.
doi: 10.1021/cr60109a003 |
|
(b) da Silva, J. F. M.; Garden, S. J.; Pinto, A. C. J. Braz. Chem. Soc. 2001, 12, 273.
doi: 10.1021/cr60109a003 |
|
| [2] |
(a) Ratan, B. T.; Anand, B.; Yogeeswari, P.; Sriram, D. Bioorg. Med. Chem. Lett. 2005, 15, 4451.
doi: 10.1016/j.bmcl.2005.07.046 |
|
(b) Raj, A.; Raghunathan, R.; Sridevikumaria, M. R.; Raman, N. Bioorg. Med. Chem. 2003, 11, 407.
doi: 10.1016/j.bmcl.2005.07.046 |
|
|
(c) Verma, M.; Pandeya, S. N.; Singh, K. N.; Stables, J. P. Acta Pharm. 2004, 54, 49.
doi: 10.1016/j.bmcl.2005.07.046 |
|
|
(d) Jiang, T.; Kuhen, K. L.; Wolff, K.; Yin, H.; Bieza, K.; Caldwell, J.; Bursulaya, B.; Tuntlad, T.; Zhang, K.; Karanewsky, D.; He, Y. Bioorg. Med. Chem. Lett. 2006, 16, 2109.
doi: 10.1016/j.bmcl.2005.07.046 |
|
|
(e) Aboul-Fadl, T.; Bin-Jubair, F. A. S. Int. J. Res. Pharm. Sci. 2010, 1, 113.
doi: 10.1016/j.bmcl.2005.07.046 |
|
|
(f) Sharma, S.; Gupta, M. K.; Saxena, A. K.; Bedi, P. M. S. Bioorg. Med. Chem. 2015, 23, 7165.
doi: 10.1016/j.bmcl.2005.07.046 |
|
|
(g) Harbinder, S.; Jatinder, V. S.; Gupta, M. K.; Sharma, S.; Nepali, K.; Bedi, P. M. S. Bioorg. Med. Chem. Lett. 2017, 27, 3974.
doi: 10.1016/j.bmcl.2005.07.046 |
|
| [3] |
Sandmeyer T. Helv. Chim. Acta 1919, 2 234.
doi: 10.1002/hlca.19190020125 |
| [4] |
(a) Stollé, R. Ber. Dtsch. Chem. Ges. 1913, 46, 3915.
doi: 10.1002/(ISSN)1099-0682 |
|
(b) Stollé, R. J. Prakt. Chem. 1922, 106, 137.
doi: 10.1002/(ISSN)1099-0682 |
|
| [5] | (a) Martinet, J. Compt. Rend. 1918, 166, 85. |
| (b) Bonnefoy, J.; Martinet, J. Compt. Rend. 1921, 172, 220. | |
| [6] |
Xie Y. Chem. Commun. 2016, 52 12372.
doi: 10.1039/C6CC05769A |
| [7] |
(a) Sun, J.; Liu, B.; Xu, B. RSC Adv. 2013, 3, 5824.
doi: 10.1039/c3ra40657a |
|
(b) Liu, T.; Yang, H.; Jiang, Y.; Fu, H. Adv. Synth. Catal. 2013, 355, 1169.
doi: 10.1039/c3ra40657a |
|
|
(c) Tang, B.-X.; Song, R.-J.; Wu, C.-Y.; Liu, Y.; Zhou, M.-B.; Wei, W.-T.; Deng, G.-B.; Yin, D.-L.; Li, J.-H. J. Am. Chem. Soc. 2010, 132, 8900.
doi: 10.1039/c3ra40657a |
|
|
(d) Liu, T.; Yang, H.; Jiang, Y.; Fu, H. Adv. Synth. Catal. 2013, 355, 1169.
doi: 10.1039/c3ra40657a |
|
|
(e) Sun, J.; Liu, B.-X.; Xu, B. RSC Adv. 2013, 3, 5824.
doi: 10.1039/c3ra40657a |
|
|
(f) Ilangovan, A.; Satish, G. Org. Lett. 2013, 15, 5726.
doi: 10.1039/c3ra40657a |
|
|
(g) Huang, P. C.; Gandeepan, P.; Cheng, C. H. Chem. Commun. 2013, 49, 8540.
doi: 10.1039/c3ra40657a |
|
|
(h) Li, J.; Zheng, Y.; Yu, X. L.; Lv, S. Y.; Wang, Q. T.; Hai, L.; Wu Y. RSC Adv. 2015, 5, 103280.
doi: 10.1039/c3ra40657a |
|
|
(i) Liu, Z.; Zhang, J.; Chen, S.; Shi, E.; Xu, Y.; Wan, X. Angew. Chem., Int. Ed. 2012, 51, 3231.
doi: 10.1039/c3ra40657a |
|
|
(j) Meng, Q.; Wang, F.; Li, M. J. Mol. Model. 2013, 19, 2225.
doi: 10.1039/c3ra40657a |
|
| [8] |
Liao Y.-Y.; Gao Y.-C.; Zheng W.; Tang R.-Y. Adv. Synth. Catal. 2018, 360 3391.
doi: 10.1002/adsc.201800592 |
| [9] |
Lollar C. T.; Krenek K. M.; Bruemmer K. J.; Lippert A. R. Org. Biomol. Chem. 2014, 12 406.
doi: 10.1039/C3OB42024H |
| [10] |
(a) Satish, G.; Polu, A.; Ramar, T.; Ilangovan, A. J. Org. Chem. 2015, 80, 5167.
doi: 10.1021/acs.joc.5b00581 |
|
(b) Reddy, M. R.; Rao, N. N.; Ramakrishna, K.; Meshram, H. M. Tetrahedron Lett. 2014, 55, 4758.
doi: 10.1021/acs.joc.5b00581 |
|
|
(c) Ilangovan, A.; Satish, G. J. Org. Chem. 2014, 79, 4984.
doi: 10.1021/acs.joc.5b00581 |
|
|
(d) Gao, F. F.; Xue, W. J.; Wang, J. G.; Wu, A. X. Tetrahedron 2014, 70, 4331.
doi: 10.1021/acs.joc.5b00581 |
|
| [11] |
(a) Huang, P. C.; Gandeepan, P.; Cheng, C. H. Chem. Commun. 2013, 49, 8540.
doi: 10.1039/c3cc44435j |
|
(b) Wu, H.; Zhang, Z. G.; Liu, Q. F.; Liu, T. X.; Ma, N. N.; Zhang, G. S. Org. Lett. 2018, 20, 2897.
doi: 10.1039/c3cc44435j |
|
|
(c) Salvanna, N.; Reddy, L. M.; Kumar, R. A.; Das, B. ChemistrySelect 2018, 3, 8019.
doi: 10.1039/c3cc44435j |
|
| [12] |
Nobrega J. A.; Goncalves S. M. C.; Peppe C. Synth. Commun. 2002, 32 3711.
doi: 10.1081/SCC-120015383 |
| [13] |
Shekhar A. C.; Kumar A. R.; Sathaiah G.; Paul V. L.; Sridhar M.; Rao P. S. Tetrahedron Lett. 2009, 50 7099.
doi: 10.1016/j.tetlet.2009.10.006 |
| [14] |
Kirincich S. J.; Xiang J.; Green N.; Tam S.; Yang H. Y.; Shim J.; Clark J. D.; McKew J. C. Bioorg. Med. Chem. 2009, 17 4383.
doi: 10.1016/j.bmc.2009.05.027 |
| [15] |
Luo J. F.; Gao S. S.; Ma Y. R.; Ge G. P. Synlett 2018, 29 969.
doi: 10.1055/s-0036-1591904 |
| [16] |
Ji H. H.; Zhu Y. Z.; Shao Y.; Liu J.; Yuan Y.; Jia X. D. J. Org. Chem. 2017, 82 9859.
doi: 10.1021/acs.joc.7b01480 |
| [17] |
Wang H. Y.; Wang K. Y.; Man Y. Q.; Gao X. N.; Yang L. M.; Ren Y. F.; Li N.; Tang B.; Zhao G. Adv. Synth. Catal. 2017, 359 3934.
doi: 10.1002/adsc.201700649 |
| [18] |
Bredenkampa A.; Mohrb F.; Kirsch S. F. Synthesis 2015, 47 1937.
doi: 10.1055/s-0034-1380517 |
| [19] |
Zhang C.; Li S.; Filip B.; Richmond L.; Ye X.; Jiang Z. ACS Catal. 2016, 6 10 6853.
doi: 10.1021/acscatal.6b01969 |
| [20] |
Salvanna N.; Ramesh P.; Kumarc K. S.; Das B. New J. Chem. 2017, 41 13754.
doi: 10.1039/C7NJ02441J |
| [1] | Xiaoyang Gao, Ruirui Zhai, Xun Chen, Shuojin Wang. Recent Progress in C—H Bond Activation Reaction with Vinylene Carbonate [J]. Chinese Journal of Organic Chemistry, 2023, 43(9): 3119-3134. |
| [2] | Yanhua Gao, Yinpan Zhang, Yan Zhang, Tao Song, Yong Yang. Visible-Light-Induced Aerobic Oxidation of Alcohols over Surface Oxygen Vacancies-Enriched Nb2O5 [J]. Chinese Journal of Organic Chemistry, 2023, 43(7): 2572-2579. |
| [3] | Zeren Sun, Bingxin Zhai, Guangchao He, Hui Shen, Linya Chen, Shan Zhang, Yi Zou, Qihua Zhu, Yungen Xu. Synthesis and Anti-inflammatory Evaluation of Novel 1,2,3-Triazole Derivatives [J]. Chinese Journal of Organic Chemistry, 2023, 43(6): 2143-2155. |
| [4] | Kongchuan Wu, Kaihong Lu, Jianbin Lin, Huijun Zhang. Research Progress in Ortho-C—H Bond Functionalization of Rylene Diimides [J]. Chinese Journal of Organic Chemistry, 2023, 43(3): 1000-1011. |
| [5] | Ni Wang, Zijun Zheng, Xiaoping Jia, Mengyuan Zhao, Yalei Wang, Chen Zhou, Zhijia Wang, Zelin Xiao, Hongmin Liu, Yu Ke. Study on Synthesis and Pharmacological Research of Jiyuan Oridonin A Derivatives as Potential Anti-tumor Drugs [J]. Chinese Journal of Organic Chemistry, 2023, 43(2): 646-659. |
| [6] | Fang Wang, Lei Wang. Recent Advances in Functionalization of Aromatic C(sp2)—H Bonds Based on N-Nitroso Direction [J]. Chinese Journal of Organic Chemistry, 2023, 43(12): 4157-4167. |
| [7] | Juan Tang, Jiayu Hu, Zhiqiang Zhu, Shouzhi Pu. Recent Advances in Visible-Light-Induced Organic Phosphine- Promoted Deoxygenative Functionalization Reactions [J]. Chinese Journal of Organic Chemistry, 2023, 43(12): 4036-4056. |
| [8] | Meijiao Sun, Jing Tan, Yu Tan, Jinsong Peng, Chunxia Chen. Pd-Catalyzed C(2)—H Arylation of 3-(2-Aminopyrimidin-4-yl)indoles [J]. Chinese Journal of Organic Chemistry, 2023, 43(11): 3945-3959. |
| [9] | Runhong Jia, Shuai Liu, Shichao Wang, Wenjuan Hao, Bo Jiang. A Solvent-Regulated Dediazotized Oxygenation of α-Aryl α-Diazoesters and 2,3-Dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) [J]. Chinese Journal of Organic Chemistry, 2022, 42(9): 2814-2822. |
| [10] | Jing Shi, Pengfei Guo, Wei Li, Haijing Sun, Lingwu Meng, Xinli Tong. Copper(I)-Catalyzed Aerobic Oxidative Condensation of Biomass-Based Platform Compound Furfurals with Straight-Chain Alcohols [J]. Chinese Journal of Organic Chemistry, 2022, 42(3): 905-909. |
| [11] | Fufang Wu, Xuejian Li, Hao Jia, Xuanzhen Han, Xiaobao Shen. Iodine(III)-Promoted Oxidative Cross-Coupling Reactions of C—H Bonds via a Free Radical Process [J]. Chinese Journal of Organic Chemistry, 2022, 42(3): 884-890. |
| [12] | Kexin Li, Qingyuan Yang, Pengpeng Zhang, Wuyuan Zhang. Research Progress of Peroxygenase-Catalyzed Reactions Driven by in-situ Generation of H2O2 [J]. Chinese Journal of Organic Chemistry, 2022, 42(3): 732-741. |
| [13] | Xiaowei Zhao, Ziqin Xia, Man Zhang, Nengneng Zhou. Radical-Mediated Tandem Cyclization to Construct Seven-Membered Nitrogen/Oxygen Heterocycles [J]. Chinese Journal of Organic Chemistry, 2022, 42(12): 3995-4023. |
| [14] | Hong'en Cao, Peizi Li, Xiaobi Jing, Hongwei Zhou. Selective Epoxidation of β-Ionone Catalyzed by Iron-Doped Se/C [J]. Chinese Journal of Organic Chemistry, 2022, 42(11): 3890-3895. |
| [15] | Yuliang Qian, Lan Xu, Yihan Tong, Tianyi Liu, Yingying Xia, Dingmao Hu, Liangce Rong. Synthesis of 2-Substituted Cyclopenta[c]chromene Derivatives by α-C Insertion of Acetonitrile (Acetone) [J]. Chinese Journal of Organic Chemistry, 2022, 42(1): 181-189. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||