

Chinese Journal of Organic Chemistry >
Iron/O2-Promoted C-H Bond Functionalization for the Exclusive Synthesis of 2-Quinoline Carboxaldehydes under Microwave Irradiation
Received date: 2019-03-04
Online published: 2019-07-09
Supported by
the National Natural Science Foundation of China(21576239)
An one-pot iron-catalyzed oxidative formylation of 2-methylquinolines to produce 2-quinoline carboxaldehydes under microwave irradiation has been achieved by employing O2 as the oxygen donor. The reaction was general for the substrates with a wide range of functional groups, providing a yield of 48%~80%. The preliminary mechanistic studies revealed that the reaction underwent a radical pathway. Advantages of this method include the easy operation, short reaction time and good selectivity.
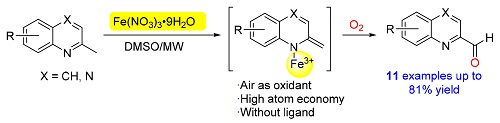
Tinghui Xie , Xiaoying Jiang , Zhisheng Mi , Xue Li , Xiaohe Xu , Renren Bai , Qi Shuai , Yuanyuan Xie . Iron/O2-Promoted C-H Bond Functionalization for the Exclusive Synthesis of 2-Quinoline Carboxaldehydes under Microwave Irradiation[J]. Chinese Journal of Organic Chemistry, 2019 , 39(11) : 3294 -3298 . DOI: 10.6023/cjoc201903007
| [1] | (a) Li, Z.; Wu, S.-S.; Luo, Z.-G.; Liu, W.-K.; Feng, C.-T.; Ma, S.-T. J. Org. Chem. 2016, 81, 4386. |
| [1] | (b) Xu, L.-B.; Shao, Z.-Z.; Wang, L.; Zhao, H.-L.; Xiao, J. Tetrahedron Lett. 2014, 55, 6856. |
| [1] | (c) Thirunavukkarasu, V. S.; Kozhushkov S. I.; Ackermann, L. Chem. Commun. 2014, 50, 29. |
| [2] | (a) Derong, D.; Linda, P. D.; Peter, A. C. Tetrahedron Lett. 2013. 54, 5211. |
| [2] | (b) Jiang, L.; Huang, Y.-Y.; Yan, Y.-Y.; Xie, Y.-Y. Tetrahedron Lett. 2016, 57, 4149. |
| [3] | Guo S.-J. Wan G. Sun S. Jiang Y. Yu J.-T. Cheng J. Chem. Commun. 2015 51 5085. |
| [4] | Xie Y.-Y. Li L.-H. Tetrahedron Lett. 2014 55 3892. |
| [5] | (a) Adsule, S.; Barve, V.; Chen, D.; Ahmed, F.; Dou, Q. P.; Padhye, S.; Sarkar, F. H. J. Med. Chem. 2006, 49, 7242. |
| [5] | (b) Teguh, S. C.; Klonis, N.; Duffy, S.; Lucantoni, L.; Avery, V. M.; Hutton, C. A.; Baell, J. B.; Tilley, L. J. Med. Chem. 2013, 56, 6200. |
| [6] | (a) Pokhrel, L.; Kim, Y.; Nguyen, T. D. T.; Prior, A. M.; Lu, J. Y.; Chang, K. O.; Hua, D. H. Bioorg. Med. Chem. Lett. 2012, 22, 3480. |
| [6] | (b) Dai, Q.; Yu, J.-T.; Feng, X.-M.; Jiang, Y.; Yang, H.-T.; Cheng, J. Adv. Synth. Catal. 2014, 356, 3341. |
| [6] | (c) Gopinath, V. S.; Pinjari, J.; Dere, R. T.; Verma, A.; Vishwakarma, P.; Shivahare, R.; Moger, M.; Goud, P. S. K.; Ramanathan, V.; Bose, P.; Rao, M. V. S.; Gupta, S.; Puri, S. K.; Launay, D.; Martin, D. Eur. J. Med. Chem. 2013, 69, 527. |
| [7] | Holzapfel C. W. Ferreira A. C. Marais W. J. Chem. Res., Synop. 2002 5 218. |
| [8] | Ding D. Dwoskin L. P. Crooks P. A. Tetrahedron Lett. 2013 54 5211. |
| [9] | Minisci F. Vismara E. Levi S. J. Org. Chem. 1986 51 536. |
| [10] | Zheng G. Liu H. Wang M. Chin. J. Chem. 2016 34 519. |
| [11] | (a) Sindhu, K. S.; Abi, T. G.; Mathai, G.; Anilkumar, G. Polyhedron 2019, 158, 270. |
| [11] | (b) Wusiman, A.; Hudabaierdi, R. Tetrahedron Lett. 2019, 60, 681. |
| [11] | (c) Wang, X.-Z.; Zeng, C.-C. Tetrahedron 2019, 75, 1425. |
| [11] | (d) Ding, X.-Y.; Xu, F. Chin. J. Org. Chem. 2018, 38, 3345 (in Chinese). |
| [11] | (丁晓友, 徐凡, 有机化学, 2018, 38, 3345.) |
| [12] | (a) Ma, S.-M.; Liu, J.-X.; Li, S.-H.; Chen, B.; Cheng, J.-J.; Kuang, J.-Q.; Liu, Y.; Wan, B.-Q.; Wang, Y.-L.; Ye, J.-T.; Yu, Q.; Yuan, W. M.; Yu, S.-C. Adv. Synth. Catal. 2011, 353, 1005. |
| [12] | (b) Silva, M. J.; Carari, D. M. Catal. Lett. 2014, 144, 615. |
| [12] | (c) Tanaka, S.; Kon, Y.; Uesaka, Y.; Morioka, R.; Tamura, M.; Sato, K. Chem. Lett. 2016, 45, 188. |
| [12] | (d) Xu, X.-H.; Sun, J.; Cheng, J.-Y.; Li, P.-P.; Jiang, X.-Y.; Bai, R.-R.; Xie, Y.-Y. Eur. J. Org. Chem. 2017, 47, 7160. |
| [12] | (e) Amaya, T.; Fujimoto, H. Tetrahedron Lett. 2018, 59, 2657. |
| [12] | (f) Wen, J.; Zhang, J.; Chen, S.-Y.; Li, J.; Yu, X.-Q. Angew. Chem. Int. Ed. 2008, 47, 8897. |
| [12] | (g) Namboodiri, V. V.; Polshettiwar, V.; Varma, R. S. Tetrahedron Lett. 2007, 48, 8839. |
| [13] | (a) Jiang, K.; Pi, D.-W.; Zhou, H.-F.; Liu, S.-S.; Zou, K. Tetrahedron. 2014, 70, 3056. |
| [13] | (b) Rao, N. N.; Meshram, H. M.; Tetrahedron Lett. 2013, 54, 1315. |
| [13] | (c) Li, Q.; Huang, Y.; Chen, T.-Q.; Zhou, Y.-B.; Xu, Q.; Yin, S.-F.; Han, L.-B. Org. Lett. 2014, 16, 3672. |
| [14] | Yasunari M. Kanoko Y. Takashi T. Takayuki A. Tomohiro M. Kosaku H. Hironao S. Heterocycles 2010 80 737. |
| [15] | Mathes W. Sauermilch W. Chem. Ber. 1957 90 758. |
| [16] | Chen X.-Y. Shi J. Li Y.-M. Wang F.-L. Wu X. Guo Q.-X. Liu L. Org. Lett. 2009 11 4421. |
| [17] | Brown B. R. Hammick D. L. J. Chem. Soc. 1950 628. |
| [18] | Buehler C. A. Edwards S. P. J. Am. Chem. Soc. 1952 74 4 977. |
| [19] | Ballesteros G. R. Leroux F. R. Ballesteros R. Tetrahedron 2009 65 22 4410. |
| [20] | Janina, B. PL 56421, 1968[Chem. Abstr. 1968, 70, 115023]. |
| [21] | Wang L. Hou X.-B. Fu H.-S. Pan X.-L. Xu W.-F. Tang W.-P Fang H. Bioorg. Med. Chem. 2015 15 23 4364. |
| [22] | Leese C. L. Rydon H. N. J. Chem. Soc. 1956 303. |
/
| 〈 |
|
〉 |