

Chinese Journal of Organic Chemistry >
Study on Bioactive Secondary Metabolites from Penicillium herquei JX4
Received date: 2020-09-16
Revised date: 2020-10-14
Online published: 2020-11-04
Supported by
Natural Science Foundation of Hainan Province(218QN234); Open Research Projects of Hainan Provincial Key Laboratory of Research and Development on Tropical Herbs(KF202003); National Natural Science Foundation of China(21662012); National Natural Science Foundation of China(41866005); Innovative Research Team Project of Ministry(IRT-16R19)
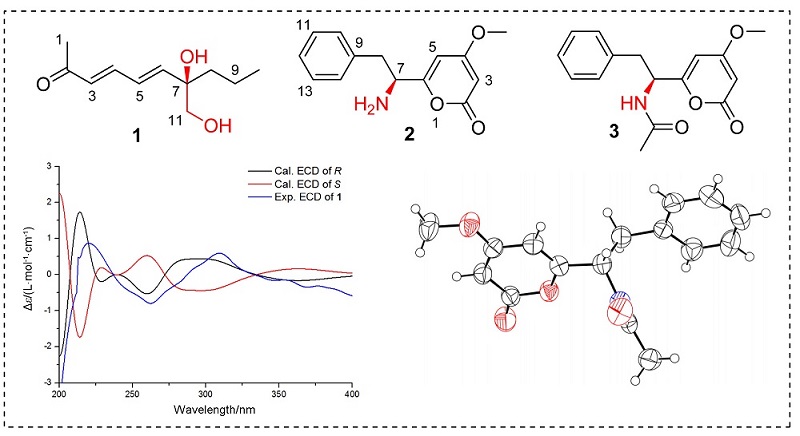
Two new secondary metabolites, penicillqueies A and B (1and2), together with penicillquei A (1), penicillquei B (2), pyrophen (3), alaromydien A (4), penicillar E (5), aculene D (6), sordaricin (7), BE-31405 (8), vermistatin (9), aspergillumarin A (10) and aspergillumarin B (11) were isolated from Penicillium herqueiJX4. Their structures were elucidated using comprehensive spectroscopic methods. The absolute configuration of penicillquei A was determined by its experimental and calculated electronic circular dichroism (ECD) spectra. Compounds 2,3, 7 and 8 showed broad spectrum antifungal activities against nine phytopathogenic fungi.
Key words: Penicillium herquei; secondary metabolites; antifungal activity
Xibin Wu , Yinfeng Tan , Jiling Yi , Xinming Song , Jingyu Yang , Xueming Zhou , Guangying Chen . Study on Bioactive Secondary Metabolites from Penicillium herquei JX4[J]. Chinese Journal of Organic Chemistry, 2021 , 41(3) : 1251 -1254 . DOI: 10.6023/cjoc202009038
| [1] | Carroll, A. R.; Copp, B. R.; Davis, R. A.; Keyzers, R. A.; Prinsep, M. R. Nat. Prod. Rep. 2020, 37, 175. |
| [2] | Rateb, M. E.; Ebel, R. Nat. Prod. Rep. 2011, 28, 290. |
| [3] | Zhang, H. W.; Song, Y. C.; Tan, R. X. Nat. Prod. Rep. 2006, 23, 753. |
| [4] | Liang, Z. Y.; Shen, N. X.; Zhen, Y. Y.; Wu, J. T.; Miao, L.; Fu, X. M.; Chen, M.; Wang, C. Y. Bioorg. Chem. 2019, 93, 103331. |
| [5] | Xue, J. H.; Li, H. X.; Wu, P.; Xu, L. X.; Yuan, Y. F.; Wei, X. Y. J. Nat. Prod. 2020, 83, 1480. |
| [6] | Zhang, D. W.; Zhao, L. L.; Wang, L. N.; Fang, X. M.; Zhao, J. Y.; Wang, X. W.; Li, L.; Liu, H. Y.; Wei, Y. Z.; You, X. F.; Cen, S.; Yu, L. Y. J. Nat. Prod. 2017, 80, 371. |
| [7] | Tang, J. L.; Zhou, Z. Y.; Yang, T.; Yao, C.; Wu, L. W.; Li, G. Y. J. Agric. Food Chem. 2019, 67, 2175. |
| [8] | Zhou, X. M.; Zheng, C. J.; Song, X. M.; Tang, M. M.; Yang, J. Y.; Yang, X.; Peng, S. J.; Cai, J.; Chen, G. Y. Fitoterapia 2019, 139, 104400. |
| [9] | Varoglu, M.; Crews, P. J. Nat. Prod. 2000, 63, 41. |
| [10] | Ma, M. Z.; Yi, W. W.; Qin, L.; Lian, X. Y.; Zhang, Z. Z. 2020, Nat. Prod. Res. DOI: 10.1080/14786419.2020.1779265. |
| [11] | Kong, F. D.; Zhou, L. M.; Ma, Q. Y.; Huang, S. Z.; Wang, P.; Dai, H. F. Phytochem. Lett. 2016, 17, 59. |
| [12] | Gao, Y. Q.; Guo, C. J.; Zhang, Q.; Zhou, W. M.; Wang, C. C. C.; Gao, J. M. Molecules 2015, 20, 325. |
| [13] | Pongcharoen, W.; Rukachaisirikul, V.; Phongpaichit, S.; Kühn, T.; Pelzing, M.; Sakayaroj, J.; Taylor, W. C. Phytochemistry 2008, 69, 1900. |
| [14] | Okada, H.; Kamiya, S.; Shiina, Y.; Suwa, H.; Nagashima, M.; Nakajima, S.; Shimokawa, H.; Sugiyama, E.; Kongo, H.; Kojiri, K.; Suda, H. J. Antibiot. 1998, 12, 1081. |
| [15] | Xia, X. K.; Huang, H. R.; She, Z. G.; Cai, J. W.; Lan, L.; Zhang, J. Y.; Fu, L. W.; Vrijmoed, L.L.P.; Lin, Y. C. Helv. Chim. Acta 2007, 90, 1925. |
| [16] | Li, S. D.; Wei, M. Y.; Chen, G. Y.; Lin, Y. C. Chem. Nat. Compd. 2012, 48, 371. |
| [17] | Zhou, X. M.; Zheng, C. J.; Chen, G. Y.; Song, X. P.; Han, C. R.; Li, G. N.; Fu, Y. H.; Chen, W. H.; Niu, Z. G. J. Nat. Prod. 2014, 77, 2021. |
/
| 〈 |
|
〉 |