

Chinese Journal of Organic Chemistry >
Iron(0)-Mediated Henry-Type Reaction of Bromonitromethane with Aldehydes for the Efficient Synthesis of 2-Nitro-alkan-1-ols
Received date: 2021-07-23
Revised date: 2021-08-27
Online published: 2021-08-29
Supported by
Nanjing Tech University(39837146); National Natural Science Foundation of China(22001121); Natural Science Foundation of Jiangsu Province(BK20180690)
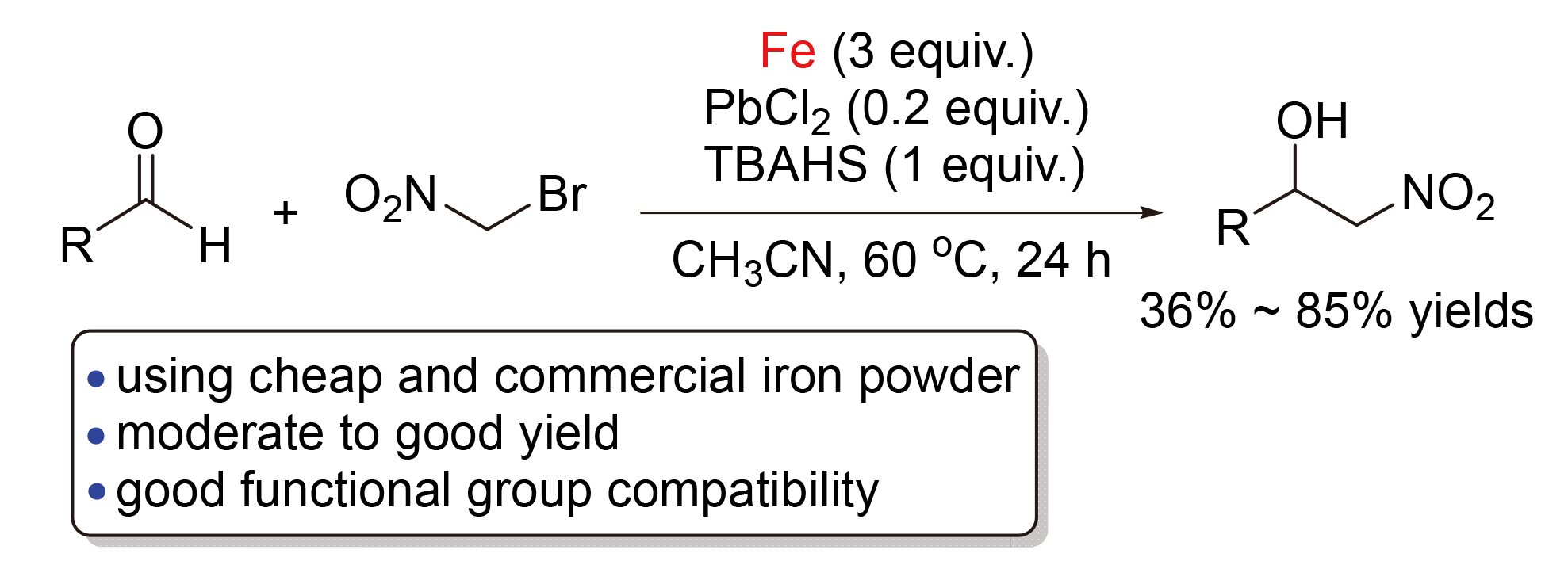
The Henry-type reaction of bromonitromethane with various aldehydes for the efficient synthesis of 2-nitro-alkan- 1-ols by using inexpensive and commercial iron powder as reaction mediator was developed. The reaction proceeded efficiently in the presence of PbCl2 and tetrabutylammonium hydrogen sulfate (TBAHS) to produce the desired products in moderate to good yield with wide functional group tolerance.
Key words: iron; lead dichloride; Henry-type reaction; bromonitromethane; 2-nitro-alkan-1-ol
Sixuan Zhang , Xiangrui Li , Wenxin Li , Weidong Rao , Danhua Ge , Zhiliang Shen , Xueqiang Chu . Iron(0)-Mediated Henry-Type Reaction of Bromonitromethane with Aldehydes for the Efficient Synthesis of 2-Nitro-alkan-1-ols[J]. Chinese Journal of Organic Chemistry, 2022 , 42(1) : 235 -241 . DOI: 10.6023/cjoc202107048
| [1] | (a) Ono, N. In The Nitro Group in Organic Synthesis, Wiley-VCH, New York, 2001, p. 30. |
| [1] | (b) Norman, B. H.; Morris, M. L. Tetrahedron Lett. 1992, 33, 6803. |
| [1] | (c) Kawabata, T.; Kiryu, Y.; Sugiure, Y.; Fuji, K. Tetrahedron Lett. 1993, 34, 5127. |
| [1] | (d) Sasai, H.; Kim, W.-S.; Suzuki, T.; Shibasaki, M. Tetrahedron Lett. 1994, 35, 6123. |
| [1] | (e) Corey, E. J.; Zhang, F.-Y. Angew. Chem. Int. Ed. 1999, 38, 1931. |
| [1] | (f) Grembecka, J.; Kafarski, P. Mini Rev. Med. Chem. 2001, 1, 133. |
| [2] | Henry, L. Bull. Soc. Chim. Fr. 1895, 13, 999. |
| [3] | (a) Rosini, G.; Ballini, R. Synthesis 1988, 833. |
| [3] | (b) Shvekhgeimer, M. A. Russ. Chem. Rev. 1998, 67, 35. |
| [3] | (c) Luzzio, F. A. Tetrahedron 2001, 57, 915. |
| [4] | (a) Concellon, J. M.; Rodriguez-Solla, H.; Concellon, C.; Garcia-Granda, S.; Diaz, M. R. Org. Lett. 2006, 8, 5979. |
| [4] | (b) Alcaide, B.; Almendros, P.; Luna, A.; Torres, M. R. Org. Biomol. Chem. 2008, 6, 1635. |
| [4] | (c) Blay, G.; Hernandez-Olmos, V.; Pedro, J. R. Chem. Commun. 2008, 4840. |
| [4] | (d) Leighty, M. W.; Shen, B.; Johnston, J. N. J. Am. Chem. Soc. 2012, 134, 15233. |
| [4] | (e) Mao, P.; Yang, L.; Xiao, Y.; Yuan, J.; Mai, W.; Gao, J.; Zhang, X. Chin. J. Org. Chem. 2019, 39, 443. |
| [4] | (f) Hu, Z.; Jiang, G.; Zhu, Z. Gong, B.; Xie, Z.; Le, Z. Chin. J. Org. Chem. 2021, 41, 325. |
| [5] | Concellon, J. M.; Rodriguez-Solla, H.; Concellon, C. J. Org. Chem. 2006, 71, 7919. |
| [6] | (a) Soengas, R. G.; Estévez, A. M. Eur. J. Org. Chem. 2010, 5190. |
| [6] | (b) Soengas, R. G.; Estévez, A. M. Synlett 2010, 2625. |
| [6] | (c) Soengas, R. G.; Estévez, A. M. Tetrahedron Lett. 2012, 53, 570. |
| [7] | Soengas, R. G.; Silva, A. M. S. Synlett 2012, 873. |
| [8] | Mahasneh, A. S. Z. Naturforsch. 2005, 60b, 416. |
| [9] | (a) Liu, Y.; Lu, Y.; Prashad, M.; Repic, O.; Blacklock, T. J. Adv. Synth. Catal. 2005, 347, 217. |
| [9] | (b) Gao, G.; Tao, Y.; Jiang, J. Green Chem. 2008, 10, 439. |
| [9] | (c) Dey, R.; Mukherjee, N.; Ahammed, S.; Ranu, B. C. Chem. Commun. 2012, 48, 7982. |
| [9] | (d) Liu, X.-Y.; Cheng, B.-Q.; Guo, Y.-C.; Chu, X.-Q.; Rao, W.; Loh, T.-P.; Shen, Z.-L. Org. Chem. Front. 2019, 6, 1581. |
| [9] | (e) Liu, X.-Y.; Li, X.-R.; Zhang, C.; Chu, X.-Q.; Rao, W.; Loh, T.-P.; Shen, Z.-L. Org. Lett. 2019, 21, 5873. |
| [9] | (f) Chan, T. C.; Lau, C. P.; Chan, T. H. Tetrahedron Lett. 2004, 45, 4189. |
| [10] | (a) Blümke, T. D.; Chen, Y.-H.; Peng, Z.; Knochel, P. Nat. Chem. 2010, 2, 313. |
| [10] | (b) Chen, B.-Z.; Wang, C.-X.; Jing, Z.-H.; Chu, X.-Q.; Loh, T.-P.; Shen, Z.-L. Org. Chem. Front. 2019, 6, 313. |
| [11] | (a) Blümke, T. D.; Klatt, T.; Koszinowski, K.; Knochel, P. Angew. Chem. Int. Ed. 2012, 51, 9926. |
| [11] | (b) Takai, K.; Ikawa, Y. Org. Lett. 2002, 4, 1727. |
| [11] | (c) Yun, J.-J.; Zhi, M.-L.; Shi, W.-X.; Chu, X.-Q.; Shen, Z.-L.; Loh, T.-P. Adv. Synth. Catal. 2018, 360, 2632. |
| [11] | (d) Shen, L.; Zhao, K.; Doitomi, K.; Ganguly, R.; Li, Y.-X.; Shen, Z.-L.; Hirao, H.; Loh, T.-P. J. Am. Chem. Soc. 2017, 139, 13570. |
| [12] | (a) Ollevier, T. Org. Biomol. Chem. 2013, 11, 2740. |
| [12] | (b) Wu, Z.; Feng, X.-X.; Wang, Q.-D.; Yun, J.-J.; Rao, W.; Yang, J.-M.; Shen, Z.-L. Chin. Chem. Lett. 2020, 31, 1297. |
| [13] | (a) Chen, C.; Liu, P.; Luo, M.; Zeng, X. ACS Catal. 2018, 8, 5864. |
| [13] | (b) Li, Y.; Deng, G.; Zeng, X. Organometallics 2016, 35, 747. |
| [13] | (c) Yun, J.-J.; Liu, X.-Y.; Deng, W.; Chu, X.-Q.; Shen, Z.-L.; Loh, T.-P. J. Org. Chem. 2018, 83, 10898. |
| [14] | (a) Enthaler, S.; Junge, K.; Beller, M. Angew. Chem. Int. Ed. 2008, 47, 3317. |
| [14] | (b) Bauer, I.; Knölker, H.-J. Chem. Rev. 2015, 115, 3170. |
| [14] | (c) Xiong, H.; Ramkumar, N.; Chiou, M.-F.; Jian, W.; Li, Y.; Su, J.-H.; Zhang, X.; Bao, H. Nat. Commun. 2019, 10, 122. |
| [14] | (d) Wei, R.; Xiong, H.; Ye, C.; Li, Y.; Bao, H. Org. Lett. 2020, 22, 3195. |
| [14] | (e) Deng, W.; Ye, C.; Li, Y.; Li, D.; Bao, H. Org. Lett. 2019, 21, 261. |
| [14] | (f) He, Z.; Fan, M.; Xu, J.; Hu, Y.; Wang, L.; Wu, X.; Xia, C.; Liu, C. Chin. J. Org. Chem. 2019, 39, 3438. |
| [15] | (a) Takai, K.; Kakiuchi, T.; Utimoto, K. J. Org. Chem. 1994, 59, 2671. |
| [15] | (b) Cheng, B.-Q.; Zhao, S.-W.; Song, X.-D.; Chu, X.-Q.; Rao, W.; Loh, T.-P.; Shen, Z.-L. J. Org. Chem. 2019, 84, 5348. |
| [16] | (a) Cheng, B.-Q.; Zhang, S.-X.; Cui, Y.-Y.; Chu, X.-Q.; Rao, W.; Xu, H.; Han, G.-Z.; Shen, Z.-L. Org. Lett. 2020, 22, 5456. |
| [16] | (b) Chu, X.-Q.; Cheng, B.-Q.; Zhang, Y.-W.; Ge, D.; Shen, Z.-L.; Loh, T.-P. Chem. Commun. 2018, 54, 2615. |
| [16] | (c) Wang, P.; Chen, B.-Z.; Guo, Y.-C.; Rao, W.; Shen, Z.-L. Tetrahedron Lett. 2019, 60, 151288. |
| [16] | (d) Wu, L.-H.; Zhao, K.; Shen, Z.-L.; Loh, T.-P. Org. Chem. Front. 2017, 4, 1872. |
| [16] | (e) Liu, B.; Xu, X.; Huang, L.; Feng, H. Chin. J. Org. Chem. 2020, 40, 1290. |
| [16] | (f) Huang, S.; Nie, Y.; Yang, J.; Zheng, Z.; Cao, J.; Xu, Z.; Xu, L. Chin. J. Org. Chem. 2020, 40, 2018. |
| [17] | Wu, Z.; Feng, X.-X.; Wang, Q.-D.; Liu, X.-Y.; Rao, W.; Yang, J.-M.; Shen, Z.-L. Chin. Chem. Lett. 2020, 31, 391. |
| [18] | (a) Chen, B.-Z.; Zhi, M.-L.; Wang, C.-X.; Chu, X.-Q.; Shen, Z.-L.; Loh, T.-P. Org. Lett. 2018, 20, 1902. |
| [18] | (b) Liu, X.-Y.; Cheng, B.-Q.; Guo, Y.-C.; Chu, X.-Q.; Li, Y.-X.; Loh, T.-P.; Shen, Z.-L. Adv. Synth. Catal. 2019, 361, 542. |
| [19] | (a) Tay, N. E. S.; Chen, W.; Levens, A.; Pistritto, V. A.; Huang, Z.; Wu, Z.; Li, Z.; Nicewicz, D. A. Nat. Catal. 2020, 3, 734. |
| [19] | (b) Bhattacharyya, A.; Kavitha, C. V.; Ghorai, M. K.; J. Org. Chem. 2016, 81, 643. |
| [19] | (c) Kirihara, M.; Osugi, R.; Saito, K.; Adachi, K.; Yamazaki, K.; Matsushima, R.; Kimura, Y. J. Org. Chem. 2019, 84, 8330. |
/
| 〈 |
|
〉 |