

Chinese Journal of Organic Chemistry >
Rhodium-Catalyzed Directing Group-Assisted Annulation of Arene C—H Bond with Vinylene Carbonate toward Isocoumarins
Received date: 2021-06-10
Revised date: 2021-07-14
Online published: 2021-09-03
Supported by
National Natural Science Foundation of China(21971025); Advanced Catalysis and Green Manufacturing Collaborative Innovation Center; “Innovation & Entrepreneurship Talents” Introduction Plan of Jiangsu Province, the Jiangsu Key Laboratory of Advanced Catalytic Materials & Technology(BM2012110); Foundation of Wenzhou Science & Technology Bureau(W20170003)
Isocoumarins are an important pharmaceutical compounds. Many synthetic pathways have been developed, mainly focusing on the cyclization of benzoic acid or other with alkynes. Sulfinylidene benzamides were facilely prepared and developed in the chelation-assisted arene ortho-C—H functionalization, and vinylene carbonate can establish an oxidant-free catalytic system, giving carbonic acid as a sole side product. In the presence of rhodium catalyst, an annulation between sulfinylidene benzamides and vinylene carbonate was developed, proceeding with directing group-assisted ortho-C—H coupling and intramolecular alcoholysis toward nonsubstituted isocoumarins in moderate to good yields.
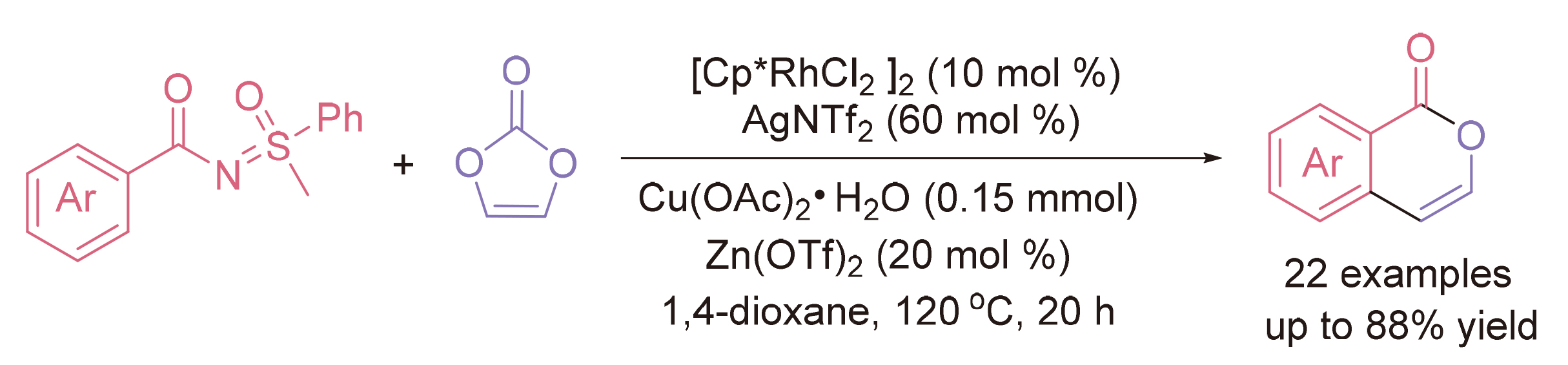
Key words: rhodium; sulfinylidene benzamides; vinylene carbonate
Lu Wang , Ying Shao , Fan Chen , Pengcheng Qian , Jiang Cheng . Rhodium-Catalyzed Directing Group-Assisted Annulation of Arene C—H Bond with Vinylene Carbonate toward Isocoumarins[J]. Chinese Journal of Organic Chemistry, 2022 , 42(1) : 242 -248 . DOI: 10.6023/cjoc202106023
| [1] | (a) Saeed, A. Eur. J. Med. Chem. 2016, 116, 290. |
| [1] | (b) Brasholz, M.; Soergel, S.; Azap, C.; Reissig, H.-U. Eur. J. Org. Chem. 2007, 3801. |
| [1] | (c) Pochet, L.; Frederick, R.; Masereel, B. Curr. Pharm. Des. 2004, 10, 3781. |
| [1] | (d) Wang, X.; Wedge, D. E.; Cutler, S. J. Nat. Prod. Commun. 2016, 11, 1595. |
| [2] | (a) Harunari, E.; Imada, C.; Igarashi, Y. J. Nat. Prod. 2019, 82, 1609. |
| [2] | (b) Das, P.; Babbar, P.; Malhotra, N.; Sharma, M.; Jachak, G. R.; Gonnade, R. G.; Shanmugam, D.; Harlos, K.; Yogavel, M.; Sharma, A.; Reddy, D. S. J. Med. Chem. 2018, 61, 5664. |
| [3] | (a) Hu, Z.; Wu, Z.; Su, Q.; Li, M.; Wu, S.; Meng, R.; Ding, W.; Li, C. Bioorg. Chem. 2020, 104, 104300. |
| [3] | (b) Zhou, J.; Zheng, L.; Hei, Z.; Li, W.; Wang, J.; Yu, B.; Fang, P. ACS Chem. Biol. 2020, 15, 1016. |
| [3] | (c) Guo, D-D.; Zhang, W.-X.; Wang, Y.-Q. Chin. J. Org. Chem. 2019, 39, 2650. (in Chinese) |
| [3] | (郭冬冬, 张武霞, 王永强, 有机化学, 2019, 39, 2650.) |
| [4] | (a) Kumar, J. S.; Thirupataiah, B.; Medishetti, R.; Ray, A.; Bele, S. D.; Hossain, K. A.; Reddy, G. S.; Edwin, R. K.; Joseph, A.; Kumar, N.; Shenoy, G. G.; Rao, C. M.; Pal, M. Eur. J. Med. Chem. 2020, 201, 112335. |
| [4] | (b) Li, X.; Zhao, C.; Jing, S.; Sun, J.; Li, X.; Man, S.; Wang, Y.; Gao, W. Bioorg. Med. Chem. Lett. 2017, 27, 3595. |
| [5] | Hu, Z.-X.; Xue, Y.-B.; Bi, X.-B.; Zhang, J.-W.; Luo, Z.-W.; Li, X.-N.; Yao, G.-M.; Wang, J.-P.; Zhang, Y.-H. Mar. Drugs 2014, 12, 5563. |
| [6] | (a) Thirupataiah, B.; Reddy, G. S.; Ghule, S. S.; Kumar, J. S.; Mounika, G.; Hossain, K. A.; Mudgal, J.; Mathew, J. E.; Shenoy, G. G.; Parsa, K. V. L.; Pal, M. Bioorg. Chem. 2020, 97, 103691. |
| [6] | (b) Yang, Z.-N.; Su, B.-J.; Wang, Y.-Q.; Liao, H.-B.; Chen, Z.-F.; Liang, D. J. Nat. Prod. 2020, 83, 985. |
| [6] | (c) Gu, L.; Liu, C.; Fang, X.; Weng, Z.; Li, Z.; Tan, Y.; Tang, K. Chin. J. Synth. Chem. 2020, 28, 841. (in Chinese) |
| [6] | (辜玲慧, 刘长英, 方新月, 翁正云, 李喆宇, 谭玉强, 唐克慧, 合成化学, 2020, 28, 841.) |
| [7] | For reviews, please see: (a) Chen, G.; Yu, Y.; Huang, X. Synlett 2018, 29, 2087. |
| [7] | (b) Saikia, P.; Gogoi, S. Adv. Synth. Catal. 2018, 360, 2063. |
| [7] | (c) Saeed, A.; Haroon, M.; Muhammad, F.; Larik, F. A.; Hesham, E.-S.; Channar, P. A. J. Organomet. Chem. 2017, 834, 88. |
| [7] | (d) Saeed, A.; Larik, F. A. Chem. Heterocycl. Compd. 2016, 52, 450. |
| [7] | (e) Ashraf, Z. Chem. Heterocycl. Compd. 2016, 52, 149. |
| [7] | (f) Pal, S.; Chatare, V.; Pal, M. Curr. Org. Chem. 2011, 15, 782. |
| [8] | For selected recently examples, please see: (a) Liu, G.; Kuang, G.; Zhang, X.; Lu, N.; Fu, Y.; Peng, Y.; Zhou, Y. Org. Lett. 2019, 21, 3043. |
| [8] | (b) Jiang, G.; Li, J.; Zhu, C.; Wu, W.; Jiang, H. Org. Lett. 2017, 19, 4440. |
| [8] | (c) Mandal, R.; Sundararaju, B. Org. Lett. 2017, 19, 2544. |
| [8] | (d) Kudo, E.; Shibata, Y.; Yamazaki, M.; Masutomi, K.; Miyauchi, Y.; Fukui, M.; Sugiyama, H.; Uekusa, H.; Satoh, T.; Miura, M.; Tanaka, K. Chem.-Eur. J. 2016, 22, 14190. |
| [8] | (e) Guo, X.-X. J. Org. Chem. 2013, 78, 166. |
| [9] | With alcohol: (a) Luo, M.-J.; Hu, M.; Song, R.-J.; He, D.-L.; Li, J.-H. Chem. Commun. 2019, 55, 1124. |
| [9] | With aldehyde: (b) Tao, L.-M.; Li, C.-H.; Chen, J.; Liu, H. J. Org. Chem. 2019, 84, 6807. |
| [9] | With aldehyde: (c) Prakash, R.; Shekarrao, K.; Gogoi, S.; Boruah, R. C. Chem. Commun. 2015, 51, 9972. |
| [10] | Mihara, G.; Ghosh, K.; Nishii, Y.; Miura, M. Org. Lett. 2020, 22, 5706. |
| [11] | For examples on the annulation of vinylene carbonate: (a) Li, X.; Huang, T.; Song, Y.; Qi, Y.; Li, L.; Li, Y.; Xiao, Q.; Zhang, Y. Org. Lett. 2020, 22, 5925. |
| [11] | (b) Ghosh, K.; Nishii, Y.; Miura, M. Org. Lett. 2020, 22, 3547. |
| [11] | (c) Ghosh, K.; Nishii, Y.; Miura, M. ACS Catal. 2019, 9, 11455. |
| [12] | (a) Raghuvanshi, K.; Zell, D.; Ackermann, L. Org. Lett. 2017, 19, 1278. |
| [12] | (b) Shankar, M.; Ghosh, K.; Mukherjee, K.; Rit, R. K.; Sahoo, A. K. Org. Lett. 2018, 20, 5144. |
| [12] | (c) Rit, R. K.; Ramu Yadav, M.; Sahoo, A. K. Org. Lett. 2012, 14, 3724. |
| [12] | (d) Ramu Yadav, M.; Rit, R. K.; Sahoo, A. K. Org. Lett. 2013, 15, 1638. |
| [12] | (e) Ramu Yadav, M.; Rit, R. K.; Sahoo, A. K. Chem.-Eur. J. 2012, 18, 5541. |
| [13] | (a) Yadav, M. R.; Rit, R. K.; Shankar, M.; Sahoo, A. K. J. Org. Chem. 2014, 79, 6123. |
| [13] | (b) Yadav, M. R.; Shankar, M.; Ramesh, E.; Ghosh, K.; Sahoo, A. K. Org. Lett. 2015, 17, 886. |
| [14] | Santhi, J.; Baire, B. Chem. Asian J. 2019, 14, 3161. |
| [15] | Toure, M.; Jaime-Figueroa, S.; Burslem, G. M.; Crews, C. M. Eur. J. Org. Chem. 2016, 4171. |
| [16] | Izumi, T.; Nishimoto, Y.; Kohei, K.; Kasahara, A. J. Heterocycl. Chem. 1990, 27, 1419. |
| [17] | Colonge, J.; Boisde, P. C. R. Hebd. Seances Acad. Sci. 1954, 239, 1047. |
| [18] | Kinder, M. A.; Kopf, J.; Margaretha, P. Tetrahedron 2000, 56, 6763. |
| [19] | Mal, D.; Bandyopadhyay, M.; Ghorai, S. K.; Datta, K. Tetrahedron Lett. 2000, 41, 3677. |
/
| 〈 |
|
〉 |