

Chinese Journal of Organic Chemistry >
Synthesis, Antifungal Activity, Three-Dimensional Quantitative Structure-Activity Relationship and Molecular Docking Study of 4-Acyl-3-amino-1,2,4-triazole-thioether Derivatives Containing Natural Pinene Structure
Received date: 2021-08-18
Revised date: 2021-10-07
Online published: 2021-11-03
Supported by
National Natural Science Foundation of China(31870556); Open Fund of Guangxi Key Laboratory of Chemistry and Engineering of Forest Products(GXFG2010)
Twenty-four 4-acyl-3-amino-1,2,4-triazole-thioether derivatives containing natural pinene structure were designed and synthesized in search of novel natural product-based bioactive molecules. Their structures were characterized by IR, 1H NMR, 13C NMR, ESI-MS, and elemental analysis. The in vitro antifungal activity test of the target compounds showed that, at the concentration of 50 µg/mL, the target compounds displayed certain antifungal activity against the eight tested plant pathogens, in which 4 compounds exhibited good to excellent antifungal activity against Physalospora piricola, much better than that of the positive control chlorothalonil. Also, 2 compounds exhibited good inhibitory activity against Cercospora arachidicola, much better than that of the positive control chlorothalonil. The preliminary analysis of three-dimensional quantitative structure-activity relationship (3D-QSAR) was carried out using the molecular field analysis (CoMFA) method for the inhibitory activity of the target compounds against P. piricola, and a reasonable 3D-QSAR model (r2=0.961, q2=0.613) has been established. Furthermore, the molecular docking results showed that the binding mode of the target compound nopol-derived 4-(4'-fluorobenzoyl)-3-amino-1,2,4-triazole-thioether (5k) in the active cavity of succinate dehydrogenase (SDH) was similar to that of the commercial fungicide carboxin.
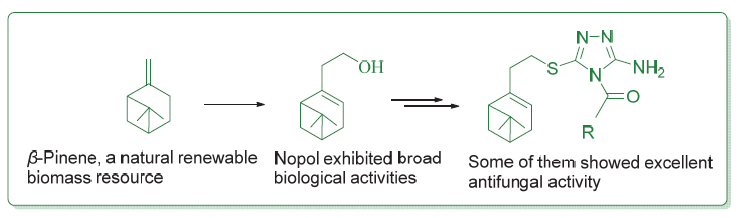
Key words: β-pinene; nopol; 1,2,4-triazole-thioether; 3D-QSAR; molecular docking; antifungal activity
Xiu Wang , Wengui Duan , Guishan Lin , Baoyu Li , Wenjing Zhang , Fuhou Lei . Synthesis, Antifungal Activity, Three-Dimensional Quantitative Structure-Activity Relationship and Molecular Docking Study of 4-Acyl-3-amino-1,2,4-triazole-thioether Derivatives Containing Natural Pinene Structure[J]. Chinese Journal of Organic Chemistry, 2022 , 42(3) : 871 -883 . DOI: 10.6023/cjoc202108031
| [1] | Wang, H. Y.; Gao, X. H.; Zhang, X. X.; Jin, H.; Tao, K.; Hou, T. P. Bioorg. Med. Chem. Lett. 2017, 27, 90. |
| [2] | Qi, L.; Li, M. C.; Bai, J. C.; Ren, Yi. H.; Ma, H. X. Bioorg. Med. Chem. Lett. 2021, 40, 127902. |
| [3] | Yu, B.; Zhou, S.; Cao, L. X.; Hao, Z. S.; Yang, D. Y.; Guo, X. F.; Zhang, N. L.; Bakulev, V. A.; Fan, Z. J. J. Agric. Food Chem. 2020, 68, 7093. |
| [4] | Yoshikawa, Y.; Katsuta, H.; Kishi, J.; Yanase, Y. J. Pestic. Sci. 2011, 36, 347. |
| [5] | FRAC classification of fungicides. http://www.frac.info/(accessed 12 Aug 2021). |
| [6] | García, D.; Bustamante, F.; Villa, A. L.; Lapuerta, M.; Alarcon, E. Energy Fuels 2020, 34, 579. |
| [7] | Nie, G. K.; Zou, J. J.; Feng, R.; Zhang, X. W.; Wang, L. Catal. Today 2014, 234, 271. |
| [8] | Xu, B.; Zhai, Q. H.; Li, Z. X.; Zheng, G. Y. Chem. Ind. Forest. Prod. 1992, 1, 75. (in Chinese) |
| [8] | (许彬, 翟其骅, 李仲训, 郑光耀, 林产化学与工业, 1992, 1, 75.) |
| [9] | Jadhav, S. V.; Jinka, K. M.; Bajaj, H. C. Appl. Catal. A: Gen. 2010, 390, 158. |
| [10] | Vrbková, E.; Štefová, B.; Vyskočilová, E.; Červený, L. React. Kinet., ech. Catal. 2020, 131, 213. |
| [11] | Chen, J. Z.; Xiao, Z. Q.; Xu, L. F.; Wang, Z. D. Chem. Res. Appl. 2017, 29, 1728. (in Chinese) |
| [11] | (陈金珠, 肖转全, 徐丽锋, 王宗德, 化学研究与应用, 2017, 29, 1728.) |
| [12] | Nyamwihura, R. J.; Zhang, H. S.; Collins, J. T.; Crown, O.; Ogungbe, I. V. Molecules 2021, 26, 1008. |
| [13] | Jin, L. L.; Xiao, Z. Q.; Chen, J. Z.; Fan, G. R.; Wang, P.; Wang, Z. D.; Chen, S. X. J. Jiangxi Normal Univ. Nat. Sci. 2015, 39, 484. (in Chinese) |
| [13] | (金霖霖, 肖转全, 陈金珠, 范国荣, 王鹏, 王宗德, 陈尚钘, 江西师范大学学报(自然科学版), 2015, 39, 484.) |
| [14] | Han, Z. J.; Wang, Z. D.; Jiang, Z. K.; Zheng, W. Q.; Qian, W. H.; Chen, J. Z.; Chen, C. Acta Agric. Univ. Jiangxiensis 2008, 30, 586. (in Chinese) |
| [14] | (韩招久, 王宗德, 姜志宽, 郑卫青, 钱万红, 陈金珠, 陈超, 江西农业大学学报, 2008, 30, 586.) |
| [15] | Han, Z. J.; Wang, Z. D.; Jiang, Z. K.; Jin, X. Y.; Qian, W. H.; Chen, C.; Chen, J. Z.; Zheng, W. Q. Chin. Bull. Entomol. 2007, 44, 863. (in Chinese) |
| [15] | (韩招久, 王宗德, 姜志宽, 金宪杨, 钱万红, 陈超, 陈金珠, 郑卫青, 昆虫知识, 2007, 44, 863.) |
| [16] | He, L. L.; Tian, Q.; Yang, Q.; Zhu, X. M.; Yang, J. S. Chem. Res. Appl. 2008, 20, 600. (in Chinese) |
| [16] | (何丽丽, 田强, 杨倩, 朱星枚, 杨劲松, 化学研究与应用, 2008, 20, 600.) |
| [17] | Chen, M.; Duan, W. G.; Lin, G. S.; Fan, Z. T.; Wang, X. Molecules 2021, 26, 1708. |
| [18] | Wang, X.; Duan, W. G.; Lin, G. S.; Chen, M.; Lei, F. H. Res. Chem. Intermed. 2021, 47, 4029. |
| [19] | Amin, N. H.; El-Saadi, M. T.; Ibrahim, A. A.; Abdel-Rahman H. M. Bioorg. Chem. 2021, 111, 104841. |
| [20] | Bitla, S.; Gayatri, A. A.; Puchakayala, M. R.; Bhukya, V. K.; Vannada, J.; Dhanavath, R.; Kuthati, B.; Kothula, D.; Sagurthi, S. R.; Atcha, K. R. Bioorg. Med. Chem. Lett. 2021, 41, 128004. |
| [21] | Shahzadi, I.; Zahoor, A. F.; Rasul, A.; Mansha, A.; Ahmad, S.; Raza, Z. ACS Omega 2021, 6, 11943. |
| [22] | Chen, Y.; Li, P.; Su, S. J.; Chen, M.; He, J.; Liu, L. W.; He, M. Wang, H.; Xue, W. RSC Adv. 2019, 9, 23045. |
| [23] | Abdel-Aziz, M.; Beshr, E. A.; Abdel-Rahman, I. M.; Ozadali, K.; Tan, O. U.; Aly, O. M. Eur. J. Med. Chem. 2014, 77, 155. |
| [24] | Peng, Z. Y.; Wang, G. C.; Zeng, Q. H.; Li, Y. F.; Wu, Y.; Liu, H. Q.; Wang, J. J.; Zhao, Y. Food Chem. 2021, 341, 128265. |
| [25] | Liao, L. P.; Jiang, C. B.; Chen, J. W.; Shi, J. G.; Li, X. H.; Wang, Y.; Wen, J.; Zhou, S. J.; Liang, J.; Lao, Y. Q.; Zhang, J. X. Eur. J. Med. Chem. 2020, 190, 112114. |
| [26] | Mustafa, M.; Anwar, S.; Elgamal, F.; Ahmed, E. R.; Aly, O. M. Eur. J. Med. Chem. 2019, 183, 111697. |
| [27] | Ghosh, S.; Liu, Y.; Garg, G.; Anyika, Mercy.; McPherson, N. T.; Ma, J.; Dobrowsky, R. T.; Blagg, B. S. J. ACS Med. Chem. Lett. 2016, 7, 813. |
| [28] | Choi, M.; Jo, H.; Kim, D.; Yun, J.; Kang, J. S.; Kim, Y.; Jung, J. K.; Hong, J. T.; Cho, J.; Kwak, J. H.; Lee, H. Arch. Pharm. Res. 2016, 39, 618. |
| [29] | Wu, W. N.; Lan, W. J.; Wu, C. Y.; Fei, Q. Front. Chem. DOI: 10.3389/fchem.2021.695628. |
| [30] | Wei, Q. Y.; Wang, X. M.; Cheng, J. H.; Zeng, G. X.; Sun, D. W. Food Chem. 2018, 268, 220. |
| [31] | Liu, J. B.; Li, Y. X.; Zhang, X. L.; Cheng, D. D.; Wei, W.; Wu, C. C.; Xie, Y. T.; Xiong, L. X.; Li, Z. M. Chin. J. Chem. 2017, 35, 368. |
| [32] | Espinoza-Moraga, M.; Singh, K.; Njoroge, M.; Kaur, G. Okombo, J.; Kock, C. D.; Smith, P. J.; Wittlin, S.; Chibale, K. Bioorg. Med. Chem. Lett. 2017, 27, 658. |
| [33] | Narsinghani, T.; Sharma, R. Chem. Pap. 2017, 71, 857. |
| [34] | Xie, Y.; Ruan, X. H.; Gong, H. Y.; Wang, Y. H.; Wang, X. B.; Zhang, J. P.; Li, Q.; Xue, W. J. Heterocycl. Chem. 2017, 54, 2644. |
| [35] | Wang, X.; Pang, F. H.; Huang, L.; Yang, X. P.; Ma, X. L.; Jiang, C. N.; Li, F. Y.; Lei, F. H. Int. J. Mol. Sci. 2018, 19, 3116. |
| [36] | Zhu, X. P.; Lin, G. S.; Duan, W. G.; Li, Q. M.; Li, F. Y.; Lu, S. Z. Molecules 2020, 25, 986. |
| [37] | Yu, Y. P.; Duan, W. G.; Lin, G. S.; Kang, G. Q.; Wang, X. Y.; Lei, F. H. Chin. J. Org. Chem. 2020, 40, 1647. (in Chinese) |
| [37] | (虞友培, 段文贵, 林桂汕, 康国强, 王晓宇, 雷福厚, 有机化学, 2020, 40, 1647.) |
| [38] | Zhao, S. Y.; Lin, G. S.; Duan, W. G.; Zhang, Q. A.; Huang, Y. L.; Lei, F. H. ACS Omega 2021, 6, 9104. |
| [39] | Liu, X. L.; Hu, T. T.; Lin, G. S.; Wang, X.; Zhu, Y.; Liang, R. Z.; Duan, W. G.; Wei, M. RSC Adv. 2020, 10, 9786. |
| [40] | Li, C. F.; Cen, B.; Duan, W. G.; Lin, G. S.; Wang, X.; Li, B. Y. Chin. J. Org. Chem. 2021, 41, 2485. (in Chinese) |
| [40] | (李成飞, 岑波, 段文贵, 林桂汕, 王秀, 李宝谕, 有机化学, 2021, 41, 2485.) |
| [41] | Yi, F. P.; Ke, M.; Wang, L. S. Chem. Ind. Forest. Prod. 2000, 20, 55. (in Chinese) |
| [41] | (易封萍, 柯敏, 王立升, 林产化学与工业, 2000, 20, 55.) |
| [42] | Balczewski, P.; Bachowska, B.; Bialas, T.; Biczak, R.; Wieczorek, W. M.; Balinäska, A. J. Agric. Food Chem. 2007, 55, 1881. |
| [43] | Huang, M.; Duan, W. G.; Lin, G. S.; Li, K.; Hu, Q. Molecules 2017, 22, 1538. |
| [44] | Dolzhenko, A. V.; Pastorin, G.; Dolzhenko, A. V.; Chui, W. K. Tetrahedron Lett. 2009, 50, 2124. |
| [45] | Takács, M.; Bubenyák, M.; Váradi, A.; Blazics, B.; Horváth, P.; Kökösi, J. Tetrahedron Lett. 2011, 52, 1863. |
| [46] | Tahlan, S.; Kumar, P.; Ramasamy, K.; Mani, V.; Mishra, R. K.; Majeed, A. B. A. Narasimhan, B. Arabian J. Chem. 2017, 10(Suppl 2), A57. |
| [47] | Wang, Z. D.; Xiao, Z. Q.; Chen, J. Z. Chem. World 2004, 45, 89. (in Chinese) |
| [47] | (王宗德, 肖转泉, 陈金珠, 化学世界, 2004, 45, 89.) |
| [48] | Yi, F. P.; Zhou, Y. H.; Li, W. G.; Liu, X. M. Chem. World 2001, 42, 93. (in Chinese) |
| [48] | (易封萍, 周永红, 李伟光, 刘雄民, 化学世界, 2001, 42, 93.) |
| [49] | Su, N. N.; Li, Y.; Yu, S. J.; Zhang, X.; Liu, X. H.; Zhao, W. G. Res Chem. Intermed. 2013, 39, 759. |
| [50] | Lin, G. S.; Bai, X.; Duan, W. G.; Cen, B.; Huang, M.; Lu, S. Z. ACS Sustainable Chem. Eng. 2019, 7, 7862. |
| [51] | Xiong, L.; Li, H.; Jiang, L. N.; Ge, J. M.; Yang, W. C.; Zhu, X. L.; Yang G. F. J. Agric. Food Chem. 2017, 65, 1021. |
| [52] | Bai, H.; Liu, X. L.; Chenzhang, P. F.; Xiao, Y. M.; Fu, B.; Qin, Z. H. Molecules 2020, 25, 5852. |
/
| 〈 |
|
〉 |