

Chinese Journal of Organic Chemistry >
Study on the Secondary Metabolites of Endophytic Penicillium sclerotiorum HLL113
Received date: 2021-09-15
Revised date: 2021-10-23
Online published: 2021-11-10
Supported by
National Natural Science Foundation of China(41866005); National Natural Science Foundation of China(22177023); Key Science and Technology Program of Hainan Province(ZDKJ202008); Innovative Research Projects of Postgraduates in Hainan Universities(Hys2020-307)
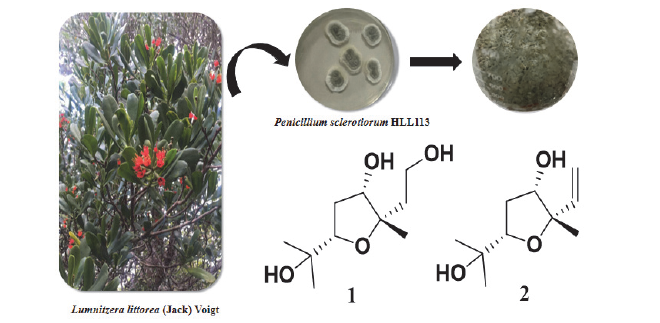
Two new furan derivatives, (2S,3S,5S)-2-(2-hydroxyethyl)-3-hydroxy-5-(2-hydroxypropan-2-yl)-2-methyltetrahydrofuran (1) and (2S,3S,5S)-2-allyl-3-hydroxy-5-(2-hydroxypropan-2-yl)-2-methyltetrahydrofuran (2), together with six known steroid compounds were isolated from Penicillium sclerotiorum HLL113. 6,9-Epoxy-ergosta-7,22-dien-3-ol (5) was isolated from the genus Penicillium for the first time. Their structures were elucidated on the basis of extensive spectroscopic methods. The test results of α-glucosidase inhibitory activity showed that (22E,4R)-ergosta-7,9(11),22-triene-3β,5α,6β-triol (7) and derrisisoflavone K (8) have significant inhibitory activity.
Jing-Yu Yang , Min-Min Tang , Li Chen , Xin-Yi Lai , Xin Zhuo , Xue-Ming Zhou , Guang-Ying Chen . Study on the Secondary Metabolites of Endophytic Penicillium sclerotiorum HLL113[J]. Chinese Journal of Organic Chemistry, 2022 , 42(3) : 896 -900 . DOI: 10.6023/cjoc202109021
| [1] | Carroll, A. R.; Copp, B. R.; Davis, R. A.; Keyzers, R. A.; Prinsep, M. R. Nat. Prod. Rep. 2021, 37, 175. |
| [2] | Cao, F.; Zhu, H.-J.; Sun, T.-T. Curr. Med. Chem. 2021, 28, 3568. |
| [3] | Song, C.-G.; Yang, J.; Zhang, M.-Z.; Ding, G.; Jia, C.-G.; Qin, J.-C.; Guo, L.-P. Chem. Biodiversity 2021, 18, e2001020. |
| [4] | Zhang, Y.; Chen, Y.-K.; Zhou, Y.; Zhang, J.-W.; Bai, H.; Zheng, C.-F. Front. Genet. 2020, 11, 584817. |
| [5] | Shi, S.-H.; Huang, Y.-L.; Zeng, K.; Tan, F.-X.; He, H.-H.; Huang, J.-Z.; Fu, Y.-X. Mol. Phylogenet. Evol. 2005, 34, 159. |
| [6] | Saad, S.; Taher, M.; Susanti, D.; Qaralleh, H.; Rahim, N. A. B. A. Asian Pac. J. Trop. Med. 2011, 7, 523. |
| [7] | Feller, I. C.; Lovelock, C. E.; Berger, U.; Mckee, K. L.; Ball, M. C. Annu. Rev. Mar. Sci. 2010, 2, 395. |
| [8] | Xu, J. RSC Adv. 2015, 5, 841. |
| [9] | Luo, Q.; Peng, C.; Ye, B.-P. Chin. J. Pharm. Biotechnol. 2016, 23, 452. (in Chinese) |
| [9] | (罗清, 彭程, 叶波平, 药物生物技术, 2016, 23, 452.) |
| [10] | Bai, M.; Zheng, C.-J.; Chen, G.-Y. J. Nat. Prod. 2021, 84, 2104. |
| [11] | Luo, Z.-W.; Tang, M.-M.; Zhou, X.-M.; Song, X.-M.; Yi, J.-L.; Zhang, B.; Yang, J.-Y.; Chen, G.-Y. Chem. Biodiversity 2021, 18, e2100027. |
| [12] | Bai, M.; Zheng, C.-J.; Nong, X.-H.; Zhou, X.-M.; Luo, Y.-P.; Chen, G.-Y. Mar. Drugs 2019, 17, 649. |
| [13] | Bai, M.; Huang, G.-L.; Mei, R.-Q.; Wang, B.; Luo, Y.-P.; Nong, X.-H.; Chen, G.-Y.; Zheng, C.-J. Mar. Drugs 2019, 17, 433. |
| [14] | Zhou, X.-M.; Zheng, C.-J.; Song, X.-M.; Tang, M.-M.; Yang, J.-Y.; Yang, X.; Peng, S.-J.; Cai, J.; Chen, G.-Y. Fitoterapia 2019, 139, 104400. |
| [15] | Bai, M.; Zheng, C.-J.; Huang, G.-L.; Mei, R.-Q.; Wang, B.; Luo, Y.-P.; Zheng, C.; Niu, Z.-G.; Chen, G.-Y. J. Nat. Prod. 2019, 82, 1155. |
| [16] | Chen, L.-L.; Wang, P.; Chen, H.-Q.; Guo, Z.-K.; Wang, H.; Dai, H.-F.; Mei, W.-L. Molecules 2017, 22, 261. |
| [17] | Abdel-lateff, A.; Fisch, K. M.; Wright, A. D.; Koenig, G. M. Planta Med. 2003, 69, 831. |
| [18] | Zhang, P.-P.; Li, Y.-F.; Jia, C.-X; Lang, J.-J.; Niaz, S.-I.; Li, J.; Yuan, J.; Yu, J.-C.; Chen, S.-H.; Liu, L. RSC Adv. 2017, 7, 49910. |
| [19] | Deng, Q.; Li, G.; Sun, M.-Y.; Yang, X.-B.; Xu, J. Nat. Prod. Res. 2020, 34, 1404. |
| [20] | Shaker, S.; Sun, T.-T.; Wang, L.-Y.; Ma, W.-Z.; Wu, D.-L.; Guo, Y.-W.; Dong, J.; Chen, Y.-X.; Zhu, L.-P.; Yang, D.-P.; Li, H.-J.; Lan, W.-J. Nat. Prod. Res. 2021, 35, 41. |
| [21] | Tian, M.-Q.; Li, J.-F.; Li, G.-H. Chem. Bioeng. 2015, 32, 29. (in Chinese) |
| [21] | (田梦青, 李建芳, 李国红, 化学与生物工程, 2015, 32, 29.) |
| [22] | Zhang, X.; Geoffroy, P.; Miesch, M.; Julien-David, D.; Raul, F.; Aoude-Werner, D.; Marchioni, E. Steroids 2005, 70, 886. |
| [23] | Sun, S.-W.; Fu, J.; Zhou, H.-N.; Zhu, T.-J.; Li, D.-H.; Mao, W.-J.; Gu, Q.-Q. Chin. J. Mar. Drugs 2014, 33, 1. (in Chinese) |
| [23] | (孙世伟, 付娟, 周会楠, 朱天骄, 李德海, 毛文君, 顾谦群, 中国海洋药物, 2014, 33, 1.) |
| [24] | Zhang, G.-W.; Ma, X.-Q.; Yan, S.-J.; Zeng, L.-M.; Su, J.-Y. Chem. J. Chin. Univ. 2005, 26, 81. (in Chinese) |
| [24] | (张广文, 马祥全, 闫素君, 曾陇梅, 苏镜娱, 高等学校化学学报, 2005, 26, 81.) |
| [25] | Ma, K.; Han, J.-J.; Bao, L.; Wei, T.-Z.; Liu, H.-W. J. Nat. Prod. 2014, 77, 942. |
| [26] | Jiang, Z.-H.; Chai, L.; Liu, Y.-P.; Qiu, H.-C.; Wang, T.-T.; Feng, X.-Y.; Wang, Y.-T.; Huang, Z.-H.; Liu, B.-M.; Fu, Y.-H. Phytochem. Lett. 2017, 22, 194. |
/
| 〈 |
|
〉 |