

Chinese Journal of Organic Chemistry >
Synthesis of Naphthalimide Derivatives as Cholinesterase Inhibitors with Aggregation Induced Emission Properties
Received date: 2021-07-30
Revised date: 2021-10-12
Online published: 2021-11-17
Supported by
National Natural Science Foundation of China(U1704176); Science and Technology Planning Project of Henan Province(212102311030); Program of Henan Vocational College of Applied Technology(2020-HJ-22)
Alzheimer’s disease (AD) is a neurodegenerative disease characterized by impaired cognitive and memory function, which seriously affects the quality of life of the elderly. At present, the main drugs for the treatment of AD are cholinesterase inhibitors, such as donepezil and rivastigmine. In this paper, a series of novel naphthimide derivatives were designed, synthesized and evaluated based on the structure of donepezil. The results showed that all of the tested compounds were acetylcholinesterase (AChE) selective inhibitors, 2-((1-(3-methoxybenzyl)piperidin-4-yl)methyl)-1H-benzo[de]isoquinoline- 1,3(2H)-dione (4k) exhibited the strongest inhibition to AChE with an IC50 value of 4.43 μmol/L, which was better than the control rivastigmine. Kinetic and molecular modeling studies indicated that 4k targeted both the catalytic active site (CAS) and the peripheral anionic site (PAS) of AChE. In addition, 4k didn’t show obvious cytotoxicity against SH-SY5Y and PC12 cell lines. Besides, these compounds exhibited typical aggregation induced emissions (AIE) properties, which might be controlled by mechanism of restriction of intramolecular rotations.
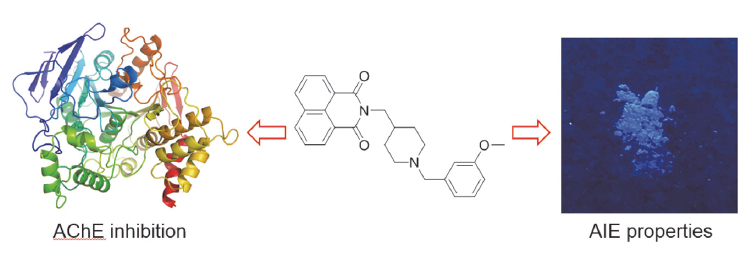
Key words: naphthalimide; cholinesterase; aggregation induced emission
Yongmei Zhao , Yeshu Mu , Wen Luo , Zhiyong Tian . Synthesis of Naphthalimide Derivatives as Cholinesterase Inhibitors with Aggregation Induced Emission Properties[J]. Chinese Journal of Organic Chemistry, 2022 , 42(3) : 819 -829 . DOI: 10.6023/cjoc202107064
| [1] | https://finance.sina.com.cn/china/gncj/2021-05-11/doc-ikmxzfmm1763811.shtml. |
| [2] | Scarpini, E.; Scheltens, P.; Feldman, H. Lancet Neurol. 2003, 2, 539. |
| [3] | Mimica, N.; Presečki, P. Psychiatr. Danub. 2009, 21, 108. |
| [4] | Du, C.-Q.; Xie, B.-H.; He, M.; Hu, Z.-Y.; Liu, Y.; He, X.; Liu, F.-Y.; Cheng, C.; Zhou, H.-B.; Huang, S.-T.; Dong, C.-E. Chin. J. Org. Chem. 2020, 40, 2035. (in Chinese) |
| [4] | (杜川黔, 谢宝花, 贺明, 胡志烨, 刘豫, 何雪, 刘凡玉, 程晨, 周海兵, 黄胜堂, 董春娥, 有机化学, 2020, 40, 2035.) |
| [5] | Zheng, S.-Y.; Yu, C.-H.; Shen, Z.-W. Chin. Chin. J. Org. Chem. 2013, 33, 2261. (in Chinese) |
| [5] | (郑书岩, 郁春辉, 沈征武, 有机化学, 2013, 33, 2261.) |
| [6] | Bortolami, M.; Rocco, D.; Messore, A.; Di Santo, R.; Costi, R.; Madia, V. N.; Scipione, L.; Pandolfi, F. Expert Opin. Ther. Pat. 2021, 31, 399. |
| [7] | Sang, Z.; Qiang, X.; Li, Y.; Xu, R.; Cao, Z.; Song, Q.; Wang, T.; Zhang, X.; Liu, H.; Tan, Z.; Deng, Y. Eur. J. Med. Chem. 2017, 135, 307. |
| [8] | Xu, Z.; Xiao, Y.; Qian, X.; Cui, J.; Cui, D. Org. Lett. 2005, 7, 889. |
| [9] | Duke, R. M.; Veale, E. B.; Pfeffer, F. M.; Kruger, P. E.; Gunnlaugsson, T. Chem. Soc. Rev. 2010, 39, 3936. |
| [10] | Xie, Z.-D.; Fu, M.-L.; Yin, B.; Zhu, Q. Chin. J. Org. Chem. 2018, 38, 1364. (in Chinese) |
| [10] | (谢振达, 付曼琳, 尹彪, 朱勍, 有机化学, 2018, 38, 1364.) |
| [11] | Tomczyk, M. D.; Walczak, K. Z. Eur. J. Med. Chem. 2018, 159, 393. |
| [12] | Stone, R. M.; Mazzola, E.; Neuberg, D.; Allen, S. L.; Pigneux, A.; Stuart, R. K.; Wetzler, M.; Rizzieri, D.; Erba, H. P.; Damon, L.; Jang, J. H.; Tallman, M. S.; Warzocha, K.; Masszi, T.; Sekeres, M. A.; Egyed, M.; Horst, H. A.; Selleslag, D.; Solomon, S. R.; Venugopal, P.; Lundberg, A. S.; Powell, B. J. Clin. Oncol. 2015, 33, 1252. |
| [13] | Chen, Z.; Xu, Y.; Qian, X. Chin. Chem. Lett. 2018, 29, 1741. |
| [14] | Tandon, R.; Luxami, V.; Kaur, H.; Tandon, N.; Paul, K. Chem. Rec. 2017, 17, 956. |
| [15] | Rong, R.-X.; Li, J.-M.; Li, Y.-F.; Guo, X.-Y.; Wang, C.; Li, Y.-J.; Li, J.-M.; Han, B.-J.; Cao, Z.-R.; Wang, K.-R.; Li, X.-L. Chin. J. Org. Chem. 2021, 41, 1599. (in Chinese) |
| [15] | (戎瑞雪, 李基民, 李耀文, 郭啸宇, 王冲, 李艳军, 李金梅, 韩宝君, 曹志然, 王克让, 李小六, 有机化学, 2021, 41, 1599.) |
| [16] | Luo, J.; Xie, Z.; Lam, J. W.; Cheng, L.; Chen, H.; Qiu, C.; Kwok, H. S.; Zhan, X.; Liu, Y.; Zhu, D.; Tang, B. Z. Chem. Commun. 2001, 18, 1740. |
| [17] | Gopikrishna, P.; Meher, N.; Iyer, P. K. ACS Appl. Mater. Interfaces 2018, 10, 12081. |
| [18] | Ma, Y.; Zhao, L.; Li, Y.; Liu, J.; Yang, Y.; Chu, T. Tetrahedron 2018, 74, 2684. |
| [19] | Srivastava, A. K.; Singh, A. K.; Kumari, N.; Yadav, R.; Gulino, A.; Speghini, A.; Nagarajan, R.; Mishra, L. J. Lumin. 2017, 182, 274. |
| [20] | Ma, X.; Chi, W.; Han, X.; Wang, C.; Liu, S.; Liu, X.; Yin, J. Chin. Chem. Lett. 2021, 32, 1790. |
| [21] | Gao, J.; Midde, N.; Zhu, J.; Terry, A. V.; McInnes, C.; Chapman, J. M. Bioorg. Med. Chem. Lett. 2016, 26, 5573. |
| [22] | Blaikie, L.; Kay, G.; Kong Thoo Lin, P. Bioorg. Med. Chem. Lett. 2020, 30, 127505. |
| [23] | Gao, J.; Suo, C.; Tseng, J. H.; Moss, M. A.; Terry, A. V., Jr.; Chapman, J. Int. J. Mol. Sci. 2021, 22, 3120. |
| [24] | Dai, F.; Li, Q.; Wang, Y.; Ge, C.; Feng, C.; Xie, S.; He, H.; Xu, X.; Wang, C. J. Med. Chem. 2017, 60, 2071. |
| [25] | Li, M.; Wang, Y.; Ge, C.; Chang, L.; Wang, C.; Tian, Z.; Wang, S.; Dai, F.; Zhao, L.; Xie, S. Eur. J. Med. Chem. 2018, 143, 1732. |
| [26] | Alonso, D.; Dorronsoro, I.; Rubio, L.; Muñoz, P.; García-Palomero, E.; Monte, M. D.; Bidon-Chanal, A.; Orozco, M.; Luque, F. J.; Castro, A.; Medina, M.; Martínez, A. Bioorg. Med. Chem. 2005, 13, 6588. |
| [27] | Ellman, G. L.; Courtney, K. D.; Andres Jr, V.; Feather-Stone, R. M. Biochem. Pharmacol. 1961, 7, 88. |
| [28] | Elsinghorst, P. W.; Tanarro, C. M.; Gutschow, M. J. Med. Chem. 2006, 49, 7540. |
| [29] | Mao, F.; Chen, J.; Zhou, Q.; Luo, Z.; Huang, L.; Li, X. Bioorg. Med. Chem. Lett. 2013, 23, 6737. |
| [30] | Sukumaran, S. D.; Nasir, S. B.; Tee, J. T.; Buckle, M. J. C.; Othman, R.; Rahman, N. A.; Lee, V. S.; Bukhari, S. N. A.; Chee, C. F. J. Enzyme Inhib. Med. Chem. 2021, 36, 130. |
/
| 〈 |
|
〉 |