

Chinese Journal of Organic Chemistry >
Recent Advances in N-Arylation of NH-Sulfoximines and Their Applications
Received date: 2021-10-09
Revised date: 2021-11-02
Online published: 2021-11-25
Supported by
Science and Technology Innovation Commission of Shenzhen Municipality(JCYJ20180302180256215)
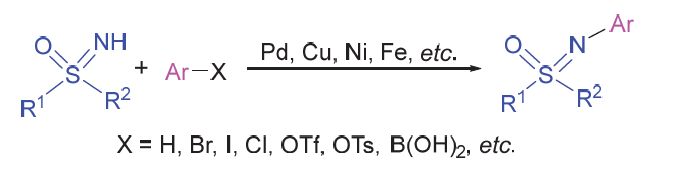
Sulfoximines represent an important structural motif in organic chemistry, and have been utilized as chiral auxiliaries, chiral ligands and organocatalysts. They also serve as key intermediates for the construction of heterocyclic compounds. Attributing to their unique bioactivities, sulfoximines have been developed as previliged pharmacophores and widely used in pharmaceutical chemistry and agriculture. Considering the wide applications, the synthetic methods to afford sulfoximines have attracted increasing attention. Among them, the direct arylation of NH-sulfoximines to prepare NAr- sulfoxmines exhibites some unique advantages, including atom-economics, mild conditions and step-economics, and thus has made tremedous progress in recent years. Various methods of NH-sulfoximines to afford NAr-sulfoximines via a C—N bond formation strategy, as well as their applications in synthesis of bioactive molecules and ligands for transition-metal catalysts, are reviewed.
Key words: NH-sulfoximine; N-arylation; NAr-sulfoximine; C—N bond formation
Xue Li , Cong Wang , Tiezheng Jia . Recent Advances in N-Arylation of NH-Sulfoximines and Their Applications[J]. Chinese Journal of Organic Chemistry, 2022 , 42(3) : 714 -731 . DOI: 10.6023/cjoc202110011
| [1] | Lücking, U. Angew. Chem., Int. Ed. 2013, 52, 9399. |
| [2] | Lücking, U.; Jautelat, R.; Krüger, M.; Brumby, T.; Lienau, P.; Schäfer, M.; Briem, H.; Schulze, J.; Hillisch, A.; Reichel, A.; Wengner, A. M.; Siemeister, G. ChemMedChem 2013, 8, 1021. |
| [3] | Zhu, Y.; Loso, M. R.; Watson, G. B.; Sparks, T. C.; Rogers, R. B.; Huang, J. X.; Gerwick, B. C.; Babcock, J. M.; Kelley, D.; Hegde, V. B.; Nugent, B. M.; Renga, J. M.; Denholm, I.; Gorman, K.; DeBoer, G. J.; Hasler, J.; Meade, T.; Thomas, J. D. J. Agric. Food Chem. 2011, 59, 2950. |
| [4] | (a) Bizet, V.; Hendriks, C. M.; Bolm, C. Chem. Soc. Rev. 2015, 44, 3378. |
| [4] | (b) Schafer, S.; Wirth, T. Angew. Chem., Int. Ed. 2010, 49, 2786. |
| [4] | (c) Tota, A.; Zenzola, M.; Chawner, S. J.; John-Campbell, S. S.; Carlucci, C.; Romanazzi, G.; Degennaro, L.; Bull, J. A.; Luisi, R. Chem. Commun. 2016, 53, 348. |
| [5] | Bolm, C.; Hildebrand, J. P. Tetrahedron Lett. 1998, 39, 5731. |
| [6] | Bolm, C.; Hildebrand, J. P. J. Org. Chem. 2000, 65, 169. |
| [7] | Harmata, M.; Hong, X. Synlett 2007, 6, 969. |
| [8] | Yongpruksa, N.; Calkins, N. L.; Harmata, M. Chem. Commun. 2011, 47, 7665. |
| [9] | Yang, Q.; Choy, P. Y.; Zhao, Q.; Leung, M. P.; Chan, H. S.; So, C. M.; Wong, W. T.; Kwong, F. Y. J. Org. Chem. 2018, 83, 11369. |
| [10] | Cho, G. Y.; Remy, P.; Jansson, J.; Moessner, C.; Bolm, C. Org. Lett. 2004, 6, 3293. |
| [11] | Sedelmeier, J.; Bolm, C. J. Org. Chem. 2005, 70, 6904. |
| [12] | Vaddula, B.; Leazer, J.; Varma, R. S. Adv. Synth. Catal. 2012, 354, 986. |
| [13] | Miyasaka, M.; Hirano, K.; Satoh, T.; Kowalczyk, R.; Bolm, C.; Miura, M. Org. Lett. 2011, 13, 359. |
| [14] | Wang, L.; Priebbenow, D. L.; Dong, W.; Bolm, C. Org. Lett. 2014, 16, 2661. |
| [15] | Grandhi, G. S.; Dana, S.; Mandal, A.; Baidya, M. Org. Lett. 2020, 22, 2606. |
| [16] | Moessner, C.; Bolm, C. Org. Lett. 2005, 7, 2667. |
| [17] | Gupta, S.; Baranwal, S.; Muniyappan, N.; Sabiah, S.; Kandasamy, J. Synthesis 2019, 51, 2171. |
| [18] | Wang, C.; Zhang, H.; Wells, L. A.; Liu, T.; Meng, T.; Liu, Q.; Walsh, P. J.; Kozlowski, M. C.; Jia, T. Nat. Commun. 2021, 12, 932. |
| [19] | Kim, J.; Ok, J.; Kim, S.; Choi, W.; Lee, P. H. Org. Lett. 2014, 16, 4602. |
| [20] | Zhu, H.; Teng, F.; Pan, C.; Cheng, J.; Yu, J.-T. Tetrahedron Lett. 2016, 57, 2372. |
| [21] | Hande, S.; Mfuh, A.; Throner, S.; Wu, Y.; Ye, Q.; Zheng, X. Tetrahedron Lett. 2019, 60, 151100. |
| [22] | Wimmer, A.; König, B. Org. Lett. 2019, 21, 2740. |
| [23] | Liu, D.; Liu, Z. R.; Ma, C.; Jiao, K. J.; Sun, B.; Wei, L.; Lefranc, J.; Herbert, S.; Mei, T. S. Angew. Chem., Int. Ed. 2021, 60, 9444. |
| [24] | Correa, A.; Bolm, C. Adv. Synth. Catal. 2008, 350, 391. |
| [25] | Wimmer, A.; König, B. Adv. Synth. Catal. 2018, 360, 3277. |
| [26] | Aithagani, S. K.; Dara, S.; Munagala, G.; Aruri, H.; Yadav, M.; Sharma, S.; Vishwakarma, R. A.; Singh, P. P. Org. Lett. 2015, 17, 5547. |
| [27] | Meier, R.; Hog, D.; Lämmermann, H.; Sudau, A.; Rackl, D.; Weinmann, H.; Collins, K.; Wortmann, L.; Candish, L. Synlett 2018, 29, 2679. |
| [28] | Harmata, M.; Pavri, N. Angew. Chem., Int. Ed. 1999, 38, 2577. |
| [29] | Harmata, M.; Ghosh, S. K. Org. Lett. 2001, 3, 3321. |
| [30] | Bolm, C.; Simic, O. J. Am. Chem. Soc. 2001, 123, 3830. |
| [31] | Bolm, C.; Martin, M.; Simic, O.; Verrucci, M. Org. Lett. 2003, 5, 427. |
| [32] | Langner, M.; Bolm, C. Angew. Chem., Int. Ed. 2004, 43, 5984. |
| [33] | Langner, M.; Remy, P.; Bolm, C. Chem.-Eur. J. 2005, 11, 6254. |
| [34] | Frings, M.; Atodiresei, I.; Wang, Y.; Runsink, J.; Raabe, G.; Bolm, C. Chem.-Eur. J. 2010, 16, 4577. |
| [35] | Moessner, C.; Bolm, C. Angew. Chem., Int. Ed. 2005, 44, 7564. |
| [36] | Biosca, M.; P?mies, O.; Diéguez, M. J. Org. Chem. 2019, 84, 8259. |
| [37] | Harmata, M.; Hong, X. J. Am. Chem. Soc. 2003, 125, 5754. |
| [38] | Harmata, M.; Hong, X. Org. Lett. 2007, 9, 2701. |
| [39] | Battula, S. R. K.; Subbareddy, G. V.; Chakravarthy, I. E.; Saravanan, V. RSC Adv. 2016, 6, 55710. |
/
| 〈 |
|
〉 |