

Chinese Journal of Organic Chemistry >
Design, Synthesis, and Antitumor Activity of β-Carboline-Benzimidazole Hybrids
Received date: 2021-10-18
Revised date: 2021-12-23
Online published: 2022-01-11
Supported by
National Natural Science Foundation of China(22067017); Project of Innovation and Development from Shihezi University(CXFZ201904); Science Foundation for The Excellent Youth Scholars of Shihezi University(RCZK201933)
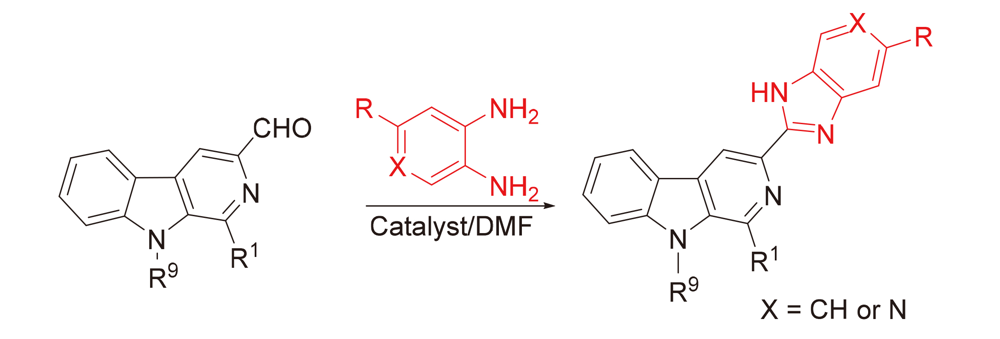
In a continuing effort to develop novel β-carboline derivatives endowed with better pharmacological profiles, a series of 1,9-disubstituted β-carboline-benzimidazole hybrids with various substituents were designed and synthesized from L-tryptophan and aldehydes. The target compounds were subjected to antitumor screening against five different cancer cell lines, namely, A549 (lung carcinoma), BGC-823 (gastric carcinoma), CT-26 (murine colon carcinoma), Bel-7402 (liver carcinoma), and MCF-7 (breast carcinoma), using standard methyl thiazolyl tetrazolium (MTT) assay. The structure-activity relationships (SARs) of the conjugates with different substituents at positions 1 and 9 in β-carbolines were also analyzed. The obtained results showed that most conjugates exhibited good cytotoxic activity against more than one cancer cell line. In particular, compound 5s with a benzyl group at position-9 and a trifluoromethyl substituent on the benzimidazole ring had the highest activity against MCF-7 cells (IC50 value of 4.9±0.3 μmol/L). Compounds 5c and 5q displayed significant cytotoxicity against three tumor cell lines, with IC50 values lower than 10 μmol/L. Moreover, these two compounds could moderately reduce the number of migrated cells at concentrations of 2.5~10 μg•mL–1.
Key words: β-carboline; benzimidazole; antitumor; structure-activity relationships
Siyu Zhu , Xinyu Huo , Qin Ma , Wei Chen , Jie Zhang , Liang Guo . Design, Synthesis, and Antitumor Activity of β-Carboline-Benzimidazole Hybrids[J]. Chinese Journal of Organic Chemistry, 2022 , 42(4) : 1129 -1135 . DOI: 10.6023/cjoc202110025
| [1] | (a) https://www.cancer.gov/about-cancer/understanding/statistics; |
| [1] | (b) http://www.who.int/mediacentre/factsheets/fs297/en. |
| [2] | Siegel, R. L.; Miller, K. D.; Jemal, A. C. A. Cancer J. Clin. 2017, 67, 7. |
| [3] | Yan, L.; Lin, M.; Pan, S.; Assaraf, Y. G.; Wang, Z. W.; Zhu, X. Drug Resistance Updates 2020, 49, 100673. |
| [4] | Chatterjee, N.; Bivona, T. G. Trends Cancer 2019, 5, 170. |
| [5] | Gaujac, A.; Navickiene, S.; Collins, M. I.; Brandt, S. D.; Andrade, J. B. Drug Test. Anal. 2012, 4, 636. |
| [6] | Xie, Z. J.; Cao, N.; Wang, C. H. Food Chem. 2021, 348, 129067. |
| [7] | Cao, R. H.; Peng, W. L.; Wang, Z. H.; Xu, A. L. Curr. Med. Chem. 2007, 14, 497. |
| [8] | Michael, C.; Robert, W. W.; Fil, G.; James, M. C.; Steven, A. B.; Kenner, C. R.; Jacqueline, N. C.; Steven, M. P., Phil, S. J. Med. Chem. 1982, 25, 1081. |
| [9] | Bournine, L.; Bensalem, S.; Fatmi, S.; Bedjou, F.; Mathieu, V.; Iguer-Ouada, M.; Kiss, R.; Duez, P. Eur. J. Integr. Med. 2017, 9, 91. |
| [10] | Sun, Y.; Guo, L.; Fan, W.-X.; Chen, W.; Zhang, J.; Dai, B. Chin. J. Org. Chem. 2021, 41, 400. (in Chinese) |
| [10] | ( 孙跃, 郭亮, 范文玺, 陈伟, 张洁, 代斌, 有机化学, 2021, 41, 400.) |
| [11] | Chen, X. F.; Guo, L.; Ma, Q.; Chen, W.; Fan, W. X.; Zhang, J. Molecules 2019, 24, 2950. |
| [12] | Kamboj, A.; Sihag, B.; Brar, D. S.; Kaur, A.; Salunke, D. B. Eur. J. Med. Chem. 2021, 221, 113536. |
| [13] | Srivastava, S. K.; Agarwal, A.; Chauhan, P. M. S.; Agarwal, S. K.; Bhaduri, A. P.; Singh, S. N.; Fatima, N.; Chatterjee, R. K. P. Bioorg. Med. Chem. 1999, 7, 1223. |
| [14] | Wang, Y. H.; Tang, J. G.; Wang, R. R.; Yang, L. M.; Dong, Z. J.; Du, L.; Shen, X.; Liu, J. K.; Zheng, Y. T. Biochem. Biophys. Res. Commun. 2007, 355, 1091. |
| [15] | Guo, L.; Xie, J. W.; Fan, W. X.; Chen, W.; Dai, B.; Ma, Q. Chin. J. Org. Chem. 2017, 37, 1741. (in Chinese) |
| [15] | ( 郭亮, 谢建伟, 范文玺, 陈伟, 代斌, 马芹, 有机化学, 2017, 37, 1741.) |
| [16] | Sun, Y.; Huo, X.-Y.; Wang, Z.-X.; Han, X.-Q.; Zhang, J. Fine Chem. 2020, 37, 1672. (in Chinese) |
| [16] | ( 孙跃, 霍新玉, 王兆旭, 韩小强, 张洁, 精细化工, 2020, 37, 1672.) |
| [17] | Huo, X. Y.; Li, W. B.; Zhang, B. Y.; Chen, X. F.; Dai, B. Chin. J. Org. Chem. 2018, 38, 3356. (in Chinese) |
| [17] | ( 霍新玉, 李文斌, 张博雅, 陈晓飞, 代斌, 有机化学, 2018, 38, 3356.) |
| [18] | Guo, L.; Chen, W.; Fan, W. X.; Ma, Q.; Sun, R. Q.; Shao, G.; Cao, R. H. Med. Chem. Commun. 2016, 7, 2177. |
| [19] | Huo, X. Y.; Guo, L.; Chen, X. F.; Zhou, Y. T.; Zhang, J.; Han, X. Q.; Dai, B. Molecules 2018, 23, 1344. |
| [20] | Narasimhan, B.; Sharma, D.; Kumar, P. Med Chem Res. 2012, 21, 269. |
| [21] | Shah, K.; Chhabra, S.; Shrivastava, S. K.; Mishra, P. Med. Chem. Res. 2013, 22, 5077. |
| [22] | Gaba, M.; Singh, S.; Mohan, C. Eur. J. Med. Chem. 2014, 76, 494. |
| [23] | Li, N.; Xin, J.-C.; Meng, Y.-Q.; Li, E.-D.; Ma, Q.-S.; Bao, C.-N. Chin. J. Org. Chem. 2018, 38, 368. (in Chinese) |
| [23] | ( 栗娜, 辛景超, 孟娅琪, 李二冬, 马启胜, 包崇男, 有机化学, 2018, 38, 368.) |
| [24] | Hsieh, C. Y.; Ko, P. W.; Chang, Y. J.; Kapoor, M.; Liang, Y. C.; Chu, H. L. Molecules 2019, 24, 3299. |
| [25] | Abonia, R.; Cortés, E.; Insuasty., B.; Insuasty, B.; Quiroga, J.; Nogueras, M.; Coboet, J. Eur. J. Med. Chem. 2011, 46, 4062. |
| [26] | Gaba, M.; Singh, S.; Mohan, C. Eur. J. Med. Chem. 2014, 76, 494. |
| [27] | (a) Sharma, D.; Narasimhan, B.; Kumar, P.; Judge, V.; Narang, R.; Clercq, E. D. J. Enzyme Inhib. Med. Chem. 2009, 24, 1161. |
| [27] | (b) Chidambaranathan, V.; Mahalakshmi, C. M. Int. J. Chem. Sci. 2015, 13, 205. |
| [28] | Karaburun, A. C.; Cavusoglu, K. C.; Cevik, U. A.; Osmaniye, D.; Sağlık, B. N.; Levent, S.; Atlı, O.; Koparal, A. S.; Kaplancıklı, Z. A. Molecules 2019, 24, 191. |
| [29] | Sridevi, C. H.; Balaji, K.; Naidu, A.; Sudhakaran, R. Eur. J. Inorg. Chem. 2012, 7, 234. |
| [30] | Chen, Z. Y.; Cao, R. H.; Shi, B. X.; Guo, L.; Sun, J.; Ma, Q.; Fan, W. X.; Song, H. C. Eur. J. Med. Chem. 2011, 46, 5127. |
| [31] | He, S.; Dobbelaar, P. H.; Guo, L.; Ye, Z.; Jian, L.; Jian, T. Biochem. Biophys. Res. Commun. 2016, 26, 1529. |
| [32] | Chen, Q.; Chen, W.; Fan, W. X.; Guo, L.; Ma, Q.; Zhang, X.D.; Du, R. L.; Cao, R. H. Bioorg. Med. Chem. Lett. 2016, 26, 5065. |
| [33] | Zhao, Y. X.; Wang, Y. Y.; Zhang, C. L.; Xu, X.; Wang, S. F. Chin. J. Org. Chem. 2021, 41, 1224. (in Chinese) |
| [33] | ( 赵雨珣, 王芸芸, 张成龙, 徐徐, 王石发, 有机化学, 2021, 41, 1224.) |
/
| 〈 |
|
〉 |