

Chinese Journal of Organic Chemistry >
Development of an Efficient Method for the Synthesis of Compounds Containing P—O—P Bonds: Pyrophosphonates, Tetraalkyl Pyrophosphates and Pyrophosphonamidates
Received date: 2022-02-07
Revised date: 2022-03-05
Online published: 2022-03-30
Supported by
State Key Laboratory of Nuclear Biological and Chemical Protection for Civilian(SKLNBC2021-09)
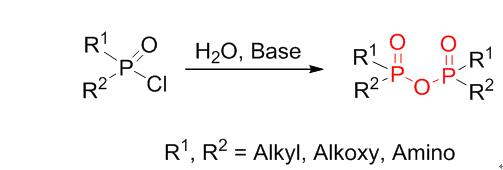
This work describes a method of synthesizing pyrophosphonates, tetraalkyl pyrophosphates and pyrophosphonamidates. Pyrophosphonates can be synthesized by the reaction of alkyl dichlorophosphonate, triethylamine (Et3N), and water, in a molar ratio of 1∶1.2∶0.5 (dichlorophosphonate∶Et3N∶water). When the reaction was allowed to proceed for 15 min in acetone, the maximum yield recorded for the pyrophosphonates was 95%. Et3N was the solvent, with an acid-binding agent. Following this method, the achieved rate of production of tetraalkyl pyrophosphate and pyrophosphonamidate was faster than the rate of production of the compounds reached using traditional methods.
Zixuan Zhang , Runli Gao , Huijuan Hu , Xiaogang Lu , Jin,Chen,Xiao Wang , Hongmei Wang . Development of an Efficient Method for the Synthesis of Compounds Containing P—O—P Bonds: Pyrophosphonates, Tetraalkyl Pyrophosphates and Pyrophosphonamidates[J]. Chinese Journal of Organic Chemistry, 2022 , 42(7) : 2214 -2221 . DOI: 10.6023/cjoc202201050
| [1] | (a) Barpanda, P.; Nishimura, S.-I.; Yamada, A. Adv. Energy Mater. 2012, 2, 841. |
| [1] | (b) Ikotun, O. F.; Marino, N.; Kruger, P. E.; Julve, M.; Doyle, R. P. Coord. Chem. Rev. 2010, 254, 890. |
| [1] | (c) Jordan, F. Nat. Prod. Rep. 2003, 20, 184. |
| [1] | (d) Kim, S. K.; Lee, D. H.; Hong, J.-I.; Yoon, J. Acc. Chem. Res. 2009, 42, 23. |
| [1] | (e) Orriss, I. R.; Arnett, T. R.; Russell, R. G. G. Curr. Opin. Pharmacol. 2016, 28, 57. |
| [2] | (a) Lin, H. Org. Biomol. Chem. 2007, 5, 2541. |
| [2] | (b) Pehar, M.; Harlan, B. A.; Killoy, K. M.; Vargas, M. R. Antioxid. Redox Signaling 2018, 28, 1652. |
| [2] | (c) Davila, A.; Liu, L.; Chellappa, K.; Redpath, P.; Nakamaru-Ogiso, E.; Paolella, L. M.; Zhang, Z.; Migaud, M. E.; Rabinowitz, J. D.; Baur, J. A. Elife 2018, 7, 1. |
| [3] | (a) McPhillips, J. J.; Coon, J. M. Toxicol. Appl. Pharmacol. 1966, 8, 66. |
| [3] | (b) Rider, J. A.; Ellinwood, L. E.; Coon, J. M. Proc. Soc. Exp. Biol. Med. 1952, 81, 455. |
| [3] | (c) Rider, J. A.; Schulman, S.; Richter, R. B.; Moeller, H. C.; DuBois, K. P. J. Am. Med. Assoc. 1951, 145, 967. |
| [4] | (a) Kimmerle, G.; Klimmer, O. R. Arch. Toxicol. 1974, 33, 1. |
| [4] | (b) Wood, S. J.; Osborne, R. H. Pestic. Sci. 1991, 32, 485. |
| [5] | (a) Wolf, R. Ind. Eng. Chem. Prod. Res. Dev. 1981, 20, 413. |
| [5] | (b) Wolf, R. Kunststoffe 1986, 76, 943. |
| [6] | (a) D'Agostino, P. A.; Provost, L. R. J. Chromatogr. 1992, 589, 287. |
| [6] | (b) D'Agostino, P. A.; Hancock, J. R.; Chenier, C. L. Eur. J. Mass Spectrom. 2003, 9, 609. |
| [6] | (c) Terent'ev, A. G.; Morozik, Y. I.; Ivanova, M. V.; Dudkin, A. V. J. Anal. Chem. 2020, 75, 208 |
| [7] | (a) Ahmadibeni, Y.; Parang, K. J. Org. Chem. 2006, 71, 5837. |
| [7] | (b) Dash, C.; Ahmadibeni, Y.; Hanley, M. J.; Pandhare, J.; Gotte, M.; Le Grice, S. F. J.; Parang, K. Bioorg. Med. Chem. Lett. 2011, 21, 3519. |
| [7] | (c) Gaines, J. C.; Ivy, E. E.; Dean, H. A.; Scales, A. L. J. Econ. Entomol. 1950, 43, 614. |
| [7] | (d) Kiran, Y. B.; Reddy, C. D.; Gunasekar, D.; Raju, C. N.; Barbosa, L. C. A.; Marney, D. C. O.; Russell, L. J. J. Fire Sci. 2007, 25, 193. |
| [7] | (e) Davidson, A.; Foley, D. A.; Frericks-Schmidt, H.; Ruggeri, S. G.; Herman, M.; LaCasse, S.; Liu, Y.; McInturff, E. L.; Morris, R.; Mugheirbi, N.; Samas, B.; Sarkar, A.; Singer, R. A.; Witkos, F.; Yu, S. Org. Process Res. Dev. 2021, 25, 621. |
| [8] | (a) Parakin, O. V.; Romakhin, A. S.; Nikitin, E. V.; Ignat'ev, Y. A.; Romanov, G. V.; Mironov, B. S.; Kargin, Y. M.; Pudovik, A. N. Zh. Obshch. Khim. 1985, 55, 2621. |
| [8] | (b) Pavlichenko, V. F.; Presnov, A. E.; Tomilov, A. P. Zh. Obshch. Khim. 1995, 65, 1347. |
| [9] | (a) Kumar, R.; Pardasani, D.; Mazumder, A.; Dubey, D. K.; Gupta, A. K. Aust. J. Chem. 2008, 61, 476. |
| [9] | (b) Kumar, R.; Gupta, A. K.; Kaushik, M. P. Phosphorus, Sulfur Silicon Relat. Elem. 2010, 185, 765. |
| [10] | (a) Toy, A. D. F. J. Am. Chem. Soc. 1948, 70, 3882. |
| [10] | (b) Toy, A. D. F. J. Am. Chem. Soc. 1950, 72, 2065. |
| [11] | Zhou, Y.; Yin, S.; Gao, Y.; Zhao, Y.; Goto, M.; Han, L.-B. Angew. Chem., Int. Ed. 2010, 49, 6852. |
| [12] | Huang, J.; He, W.; Wang, B. Phosphorus, Sulfur Silicon Relat. Elem. 2012, 187, 1125. |
| [13] | Kins, C. F.; Brunklaus, G.; Spiess, H. W. Macromolecules 2013, 46, 2067. |
| [14] | Dunning, T. H. J. Chem. Phys. 1989, 90, 1007. |
/
| 〈 |
|
〉 |